Alpha-synuclein expression in oxytocin neurons of young and old bovine brains
- PMID: 38684411
- PMCID: PMC11310384
- DOI: 10.1262/jrd.2024-020
Alpha-synuclein expression in oxytocin neurons of young and old bovine brains
Abstract
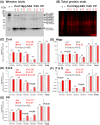
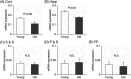
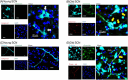
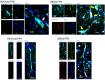
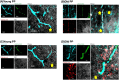
Understanding of central nervous system mechanisms underlying age-related infertility remains limited. Fibril α-synuclein, distinct from its monomeric form, is implicated in age-related diseases. Notably, fibril α-synuclein spreads among neurons, similar to prions, from damaged old neurons in cortex and hippocampus to healthy neurons. However, less is known whether α-synuclein propagates into oxytocin neurons, which play crucial roles in reproduction. We compared α-synuclein expression in the oxytocin neurons in suprachiasmatic nucleus (SCN), supraoptic nucleus (SON), paraventricular hypothalamic nucleus (PVN), and posterior pituitary (PP) gland of healthy heifers and aged cows to determine its role in age-related infertility. We analyzed mRNA and protein expression, along with Congo red histochemistry and fluorescent immunohistochemistry for oxytocin and α-synuclein, followed by confocal microscopy with Congo red staining. Both mRNA and protein expressions of α-synuclein were confirmed in the bovine cortex, hippocampus, SCN, SON, PVN, and PP tissues. Significant differences in α-synuclein mRNA expressions were observed in the cortex and hippocampus between young heifers and old cows. Western blots showed five bands of α-synuclein, probably reflecting monomers, dimers, and oligomers, in the cortex, hippocampus, SCN, SON, PVN, and PP tissues, and there were significant differences in some bands between the young heifers and old cows. Bright-field and polarized light microscopy did not detect obvious amyloid deposition in the aged hypothalami; however, higher-sensitive confocal microscopy unveiled strong positive signals for Congo red and α-synuclein in oxytocin neurons in the aged hypothalami. α-synuclein was expressed in oxytocin neurons, and some differences were observed between young and old hypothalami.
Keywords: Aging; Paraventricular hypothalamic nucleus; Posterior pituitary gland; Suprachiasmatic nucleus; Supraoptic nucleus.
Conflict of interest statement
The authors have nothing to declare.
Figures

Similar articles
-
Alpha-synuclein expression in GnRH neurons of young and old bovine hypothalami.Reprod Fertil Dev. 2024 Sep;36:RD24033. doi: 10.1071/RD24033. Reprod Fertil Dev. 2024. PMID: 39283977
-
Alpha-synuclein expression in anterior pituitary cells of aged cattle.Domest Anim Endocrinol. 2025 Jul;92:106936. doi: 10.1016/j.domaniend.2025.106936. Epub 2025 Mar 1. Domest Anim Endocrinol. 2025. PMID: 40054104
-
Vasopressin- and oxytocin-immunoreactive nerve cells in the aging rat hypothalamus.Acta Physiol Pharmacol Bulg. 1996;22(1):7-16. Acta Physiol Pharmacol Bulg. 1996. PMID: 8870838
-
Neurohypophyseal peptides in aging and Alzheimer's disease.Ageing Res Rev. 2002 Jun;1(3):537-58. doi: 10.1016/s1568-1637(02)00013-2. Ageing Res Rev. 2002. PMID: 12067600 Review.
-
Synaptic Inputs of Neural Afferent Pathways to Vasopressin- and Oxytocin-Secreting Neurons of Supraoptic and Paraventricular Hypothalamic Nuclei.Endocr Metab Immune Disord Drug Targets. 2016;16(4):276-287. doi: 10.2174/1871530317666170104124229. Endocr Metab Immune Disord Drug Targets. 2016. PMID: 28056741 Review.
References
-
- Epelbaum J, Terrien J. Mini-review: Aging of the neuroendocrine system: Insights from nonhuman primate models. Prog Neuropsychopharmacol Biol Psychiatry 2020; 100: 109854. - PubMed
-
- Osoro K, Wright IA. The effect of body condition, live weight, breed, age, calf performance, and calving date on reproductive performance of spring-calving beef cows. J Anim Sci 1992; 70: 1661–1666. - PubMed
-
- Malhi PS, Adams GP, Pierson RA, Singh J. Bovine model of reproductive aging: response to ovarian synchronization and superstimulation. Theriogenology 2006; 66: 1257–1266. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

