The genomic profiling of high-risk smoldering myeloma patients treated with an intensive strategy unveils potential markers of resistance and progression
- PMID: 38684670
- PMCID: PMC11059156
- DOI: 10.1038/s41408-024-01053-3
The genomic profiling of high-risk smoldering myeloma patients treated with an intensive strategy unveils potential markers of resistance and progression
Abstract
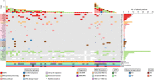
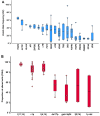
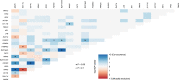
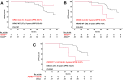
Smoldering multiple myeloma (SMM) precedes multiple myeloma (MM). The risk of progression of SMM patients is not uniform, thus different progression-risk models have been developed, although they are mainly based on clinical parameters. Recently, genomic predictors of progression have been defined for untreated SMM. However, the usefulness of such markers in the context of clinical trials evaluating upfront treatment in high-risk SMM (HR SMM) has not been explored yet, precluding the identification of baseline genomic alterations leading to drug resistance. For this reason, we carried out next-generation sequencing and fluorescent in-situ hybridization studies on 57 HR and ultra-high risk (UHR) SMM patients treated in the phase II GEM-CESAR clinical trial (NCT02415413). DIS3, FAM46C, and FGFR3 mutations, as well as t(4;14) and 1q alterations, were enriched in HR SMM. TRAF3 mutations were specifically associated with UHR SMM but identified cases with improved outcomes. Importantly, novel potential predictors of treatment resistance were identified: NRAS mutations and the co-occurrence of t(4;14) plus FGFR3 mutations were associated with an increased risk of biological progression. In conclusion, we have carried out for the first time a molecular characterization of HR SMM patients treated with an intensive regimen, identifying genomic predictors of poor outcomes in this setting.
© 2024. The Author(s).
Conflict of interest statement
The authors report personal fees from, or have consulted or served in an advisory role for Janssen (AMH, MVM, PRO, VGC, AO, LR, AA, JDLR, FDA, BP, EMO, JJL, JFSM, JML, MJC, RGS), BMS-Celgene (MVM, PRO, VGC, AO, LR, AA, JDLR, FDA, BP, EMO, JJL, JFSM, JML, MJC, RGS), Amgen (MVM, PRO, VGC, AO, LR, AA, JDLR, FDA, BP, EMO, JJL, JFSM, MJC), Takeda (MVM, AO, LR, AA, JDLR, PRO, BP, EMO, JJL, JFSM, RGS), Sanofi (MVM, AA, PRO, JDLR, FDA, BP, EMO, JJL, JFSM), GSK (MVM, PRO, AA, JDLR, FDA, EMO, BP, JFSM), Oncopeptides (MVM, AA, PRO, EMO), Regeneron (AA, EMO, MVM, PRO, JFSM), MSD (EMO, JFSM), AbbVie (EMO, PRO, MVM, JFSM), Pfizer (AA, EMO, PRO, JDLR, MVM), Roche (MVM, BP, JFSM), Karyopharm (JDLR, EMO, JFSM), Secura-Bio (EMO, JFSM), Novartis (JFSM, JML, MJC), Adaptive Biotechnologies (BP, MVM), Beckton-Dickinson (BP), Menarini-Stemline (EMO), Creative BioLabs (BP), H3 Biomedicine (PRO), Invivoscribe (AMH), Sea-Gen (MVM), Blue-Bird bio (MVM), Hospira (RGS), Pharmacyclics (RGS), Gilead (PRO, RGS) and Incyte (RGS).
Figures
References
-
- Pérez-Persona E, Vidriales MB, Mateo G, García-Sanz R, Mateos MV, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–92. doi: 10.1182/blood-2007-05-088443. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

