Emetine induces oxidative stress, cell differentiation and NF-κB inhibition, suppressing AML stem/progenitor cells
- PMID: 38684672
- PMCID: PMC11059384
- DOI: 10.1038/s41420-024-01967-8
Emetine induces oxidative stress, cell differentiation and NF-κB inhibition, suppressing AML stem/progenitor cells
Abstract
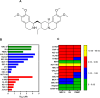
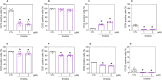
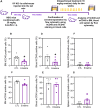
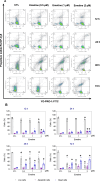
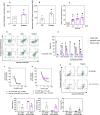
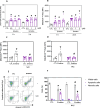
Acute myeloid leukemia (AML) is a fatal malignancy of the blood and bone marrow. Leukemic stem cells (LSCs) are a rare subset of leukemic cells that promote the development and progression of AML, and eradication of LSCs is critical for effective control of this disease. Emetine is an FDA-approved antiparasitic drug with antitumor properties; however, little is known about its potential against LSCs. Herein, we explored the antileukemic potential of emetine, focusing on its effects on AML stem/progenitor cells. Emetine exhibited potent cytotoxic activity both in hematologic and solid cancer cells and induced AML cell differentiation. Emetine also inhibited AML stem/progenitor cells, as evidenced by decreased expression of CD34, CD97, CD99, and CD123 in KG-1a cells, indicating anti-AML stem/progenitor cell activities. The administration of emetine at a dosage of 10 mg/kg for two weeks showed no significant toxicity and significantly reduced xenograft leukemic growth in vivo. NF-κB activation was reduced in emetine-treated KG-1a cells, as shown by reduced phospho-NF-κB p65 (S529) and nuclear NF-κB p65. DNA fragmentation, YO-PRO-1 staining, mitochondrial depolarization and increased levels of active caspase-3 and cleaved PARP (Asp214) were detected in emetine-treated KG-1a cells. Moreover, treatment with the pancaspase inhibitor Z-VAD(OMe)-FMK partially prevented the apoptotic cell death induced by emetine. Emetine treatment also increased cellular and mitochondrial reactive oxygen species, and emetine-induced apoptosis in KG-1a cells was partially prevented by the antioxidant N-acetylcysteine, indicating that emetine induces apoptosis, at least in part, by inducing oxidative stress. Overall, these studies indicate that emetine is a novel potential anti-AML agent with promising activity against stem/progenitor cells, encouraging the development of further studies aimed at its clinical application.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- American Cancer Society. Cancer facts & figures 2023. Atlanta: American Cancer Society; 2023.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

