Molecular Mechanisms in Metal Oxide Nanoparticle-Tryptophan Interactions
- PMID: 38684718
- PMCID: PMC11094791
- DOI: 10.1021/acs.inorgchem.3c03674
Molecular Mechanisms in Metal Oxide Nanoparticle-Tryptophan Interactions
Abstract
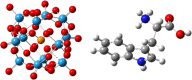
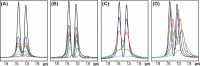
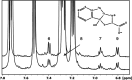
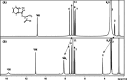
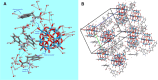
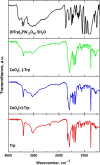
One of the crucial metabolic processes for both plant and animal kingdoms is the oxidation of the amino acid tryptophan (TRP) that regulates plant growth and controls hunger and sleeping patterns in animals. Here, we report revolutionary insights into how this process can be crucially affected by interactions with metal oxide nanoparticles (NPs), creating a toolbox for a plethora of important biomedical and agricultural applications. Molecular mechanisms in TRP-NP interactions were revealed by NMR and optical spectroscopy for ceria and titania and by X-ray single-crystal study and a computational study of model TRP-polyoxometalate complexes, which permitted the visualization of the oxidation mechanism at an atomic level. Nanozyme activity, involving concerted proton and electron transfer to the NP surface for oxides with a high oxidative potential, like CeO2 or WO3, converted TRP in the first step into a tricyclic organic acid belonging to the family of natural plant hormones, auxins. TiO2, a much poorer oxidant, was strongly binding TRP without concurrent oxidation in the dark but oxidized it nonspecifically via the release of reactive oxygen species (ROS) in daylight.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
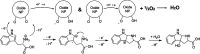
References
-
- Hrboticky N.; Leiter L. A.; Anderson G. H. Effects of L-Tryptophan on short term food intake in lean men. Nutr. Res. (N.Y.) 1985, 5, 595–607. 10.1016/S0271-5317(85)80240-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

