Baicalein triggers ferroptosis in colorectal cancer cells via blocking the JAK2/STAT3/GPX4 axis
- PMID: 38684798
- PMCID: PMC11272787
- DOI: 10.1038/s41401-024-01258-z
Baicalein triggers ferroptosis in colorectal cancer cells via blocking the JAK2/STAT3/GPX4 axis
Abstract
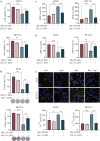
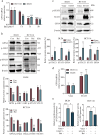
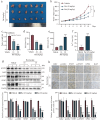
Colorectal cancer (CRC) is a prevalent form of gastrointestinal malignancy with challenges in chemotherapy resistance and side effects. Effective and low toxic drugs for CRC treatment are urgently needed. Ferroptosis is a novel mode of cell death, which has garnered attention for its therapeutic potential against cancer. Baicalein (5, 6, 7-trihydroxyflavone) is the primary flavone extracted from the dried roots of Scutellaria baicalensis that exhibits anticancer effects against several malignancies including CRC. In this study, we investigated whether baicalein induced ferroptosis in CRC cells. We showed that baicalein (1-64 μM) dose-dependently inhibited the viability of human CRC lines HCT116 and DLD1. Co-treatment with the ferroptosis inhibitor liproxstatin-1 (1 μM) significantly mitigated baicalein-induced CRC cell death, whereas autophagy inhibitor chloroquine (25 μM), necroptosis inhibitor necrostatin-1 (10 μM), or pan-caspase inhibitor Z-VAD-FMK (10 μM) did not rescue baicalein-induced CRC cell death. RNA-seq analysis confirmed that the inhibitory effect of baicalein on CRC cells is associated with ferroptosis induction. We revealed that baicalein (7.5-30 μM) dose-dependently decreased the expression levels of GPX4, key regulator of ferroptosis, in HCT116 and DLD1 cells by blocking janus kinase 2 (JAK2)/STAT3 signaling pathway via direct interaction with JAK2, ultimately leading to ferroptosis in CRC cells. In a CRC xenograft mouse model, administration of baicalein (10, 20 mg/kg, i.g., every two days for two weeks) dose-dependently inhibited the tumor growth with significant ferroptosis induced by inhibiting the JAK2/STAT3/GPX4 axis in tumor tissue. This study demonstrates that ferroptosis contributes to baicalein-induced anti-CRC activity through blockade of the JAK2/STAT3/GPX4 signaling pathway, which provides evidence for the therapeutic application of baicalein against CRC.
Keywords: GPX4; JAK2/STAT3; baicalein; colorectal cancer; ferroptosis; liproxstatin-1.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Haraldsdottir S, Einarsdottir HM, Smaradottir A, Gunnlaugsson A, Halfdanarson TR. Colorectal cancer - review. Laeknabladid. 2014;100:75–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

