A link between circulating immune complexes and acute kidney injury in human visceral leishmaniasis
- PMID: 38684845
- PMCID: PMC11059367
- DOI: 10.1038/s41598-024-60209-0
A link between circulating immune complexes and acute kidney injury in human visceral leishmaniasis
Abstract
Visceral leishmaniasis (VL) is an infectious disease caused by Leishmania infantum. Clinically, VL evolves with systemic impairment, immunosuppression and hyperactivation with hypergammaglobulinemia. Although renal involvement has been recognized, a dearth of understanding about the underlying mechanisms driving acute kidney injury (AKI) in VL remains. We aimed to evaluate the involvement of immunoglobulins (Igs) and immune complexes (CIC) in the occurrence of AKI in VL patients. Fourteen VL patients were evaluated between early treatment and 12 months post-treatment (mpt). Anti-Leishmania Igs, CIC, cystatin C, C3a and C5a were assessed and correlated with AKI markers. Interestingly, high levels of CIC were observed in VL patients up to 6 mpt. Concomitantly, twelve patients met the criteria for AKI, while high levels of cystatin C were observed up to 6 mpt. Plasmatic cystatin C was positively correlated with CIC and Igs. Moreover, C5a was correlated with cystatin C, CIC and Igs. We did not identify any correlation between amphotericin B use and kidney function markers in VL patients, although this association needs to be further explored in subsequent studies. Our data reinforce the presence of an important renal function impairment during VL, suggesting the involvement of Igs, CIC, and C5a in this clinical condition.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

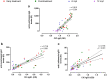

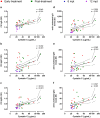


References
-
- World Health Organization. Leishmaniasis. https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (2023).
-
- Pan American Health Organization. In Leishmaniases Epidemiological Report on the Region of the Americas. https://iris.paho.org/bitstream/handle/10665.2/56831/PAHOCDEVT220021_eng... (2022).
-
- Ministério da Saúde (BR). Leishmaniose visceral—casos confirmados notificados no sistema de informação de agravos de notificação—Minas Gerais. http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/leishvmg.def (2023).
Publication types
MeSH terms
Substances
Grants and funding
- E-26/200.939/2020/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/202.944/2016/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- 302069/2022/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- Universal - 433637/2018-8/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- Pro-Ciência/2019-2022/Instituto Federal de Educação, Ciência e Tecnologia do Rio de Janeiro
LinkOut - more resources
Full Text Sources
Miscellaneous

