Glucose hypometabolism prompts RAN translation and exacerbates C9orf72-related ALS/FTD phenotypes
- PMID: 38684907
- PMCID: PMC11094177
- DOI: 10.1038/s44319-024-00140-7
Glucose hypometabolism prompts RAN translation and exacerbates C9orf72-related ALS/FTD phenotypes
Abstract
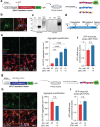
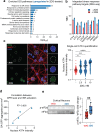
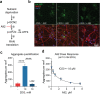
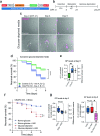
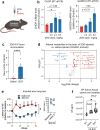
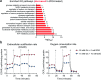
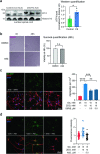
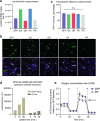
The most prevalent genetic cause of both amyotrophic lateral sclerosis and frontotemporal dementia is a (GGGGCC)n nucleotide repeat expansion (NRE) occurring in the first intron of the C9orf72 gene (C9). Brain glucose hypometabolism is consistently observed in C9-NRE carriers, even at pre-symptomatic stages, but its role in disease pathogenesis is unknown. Here, we show alterations in glucose metabolic pathways and ATP levels in the brains of asymptomatic C9-BAC mice. We find that, through activation of the GCN2 kinase, glucose hypometabolism drives the production of dipeptide repeat proteins (DPRs), impairs the survival of C9 patient-derived neurons, and triggers motor dysfunction in C9-BAC mice. We also show that one of the arginine-rich DPRs (PR) could directly contribute to glucose metabolism and metabolic stress by inhibiting glucose uptake in neurons. Our findings provide a potential mechanistic link between energy imbalances and C9-ALS/FTD pathogenesis and suggest a feedforward loop model with potential opportunities for therapeutic intervention.
Keywords: ALS; C9orf72; FTD; Glucose Hypometabolism; RAN Translation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Update of
-
Glucose Hypometabolism Prompts RAN Translation and Exacerbates C9orf72-related ALS/FTD Phenotypes.bioRxiv [Preprint]. 2023 Jun 7:2023.06.07.544100. doi: 10.1101/2023.06.07.544100. bioRxiv. 2023. Update in: EMBO Rep. 2024 May;25(5):2479-2510. doi: 10.1038/s44319-024-00140-7. PMID: 37333144 Free PMC article. Updated. Preprint.
References
-
- Ash Peter EA, Bieniek Kevin F, Gendron Tania F, Caulfield T, Lin W-L, DeJesus-Hernandez M, van Blitterswijk Marka M, Jansen-West K, Paul Joseph W, Rademakers R, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. - DOI - PMC - PubMed
-
- Beghi E, Pupillo E, Bonito V, Buzzi P, Caponnetto C, Chio A, Corbo M, Giannini F, Inghilleri M, Bella VL, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:397–405. doi: 10.3109/21678421.2013.764568. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- RF1 NS114128/NS/NINDS NIH HHS/United States
- W81XWH-21-1-0134/DOD | USA | MEDCOM | MRDC | U.S. Army Medical Research Acquisition Activity (USAMRAA)
- F31-NS118838/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS109150/NS/NINDS NIH HHS/United States
- RF1-NS114128/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- 628389/Muscular Dystrophy Association (MDA)
- R35 NS122209/NS/NINDS NIH HHS/United States
- R21 NS090912/NS/NINDS NIH HHS/United States
- RO1-NS109150/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- F31 NS118838/NS/NINDS NIH HHS/United States
- RF1-AG057882/HHS | NIH | National Institute on Aging (NIA)
- RF1 AG057882/AG/NIA NIH HHS/United States
- R21-NS090912/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

