Changes in blood metabolomes as potential markers for severity and prognosis in doxorubicin-induced cardiotoxicity: a study in HER2-positive and HER2-negative breast cancer patients
- PMID: 38685030
- PMCID: PMC11059746
- DOI: 10.1186/s12967-024-05088-9
Changes in blood metabolomes as potential markers for severity and prognosis in doxorubicin-induced cardiotoxicity: a study in HER2-positive and HER2-negative breast cancer patients
Abstract
Background: We aimed to compare the changes in blood metabolomes and cardiac parameters following doxorubicin treatment in HER2-positive and HER2-negative breast cancer patients. Additionally, the potential roles of changes in blood metabolomes as severity and prognostic markers of doxorubicin-induced cardiotoxicity were determined.
Methods: HER2-positive (n = 37) and HER2-negative (n = 37) breast cancer patients were enrolled. Cardiac function assessment and blood collection were performed at baseline and 2 weeks after completion of doxorubicin treatment in all patients, as well as at three months after completion of doxorubicin treatment in HER2-negative breast cancer patients. Blood obtained at all three-time points was processed for measuring cardiac injury biomarkers. Blood obtained at baseline and 2 weeks after completion of doxorubicin treatment were also processed for measuring systemic oxidative stress and 85 metabolome levels.
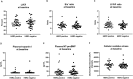
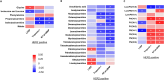
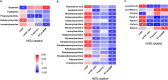
Results: Cardiac injury and systolic dysfunction 2 weeks after completion of doxorubicin treatment were comparable between these two groups of patients. However, only HER2-negative breast cancer patients exhibited increased systemic oxidative stress and cardiac autonomic dysfunction at this time point. Moreover, 33 and 29 blood metabolomes were altered at 2 weeks after completion of doxorubicin treatment in HER2-positive and HER2-negative breast cancer patients, respectively. The changes in most of these metabolomes were correlated with the changes in cardiac parameters, both at 2 weeks and 3 months after completion of doxorubicin treatment.
Conclusions: The changes in blood metabolomes following doxorubicin treatment were dependent on HER2 status, and these changes might serve as severity and prognostic markers of doxorubicin-induced cardiotoxicity.
Trial registration: The study was conducted under ethical approval from the Institutional Review Board of the Faculty of Medicine, Chiang Mai University (Registration number: MED-2563-07001; Date: April 28, 2020). The study also complied with the Declaration of Helsinki.
Keywords: Breast cancer; Cardiotoxicity; Doxorubicin; HER2; Heart failure; Metabolomes.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

