Blocking the MIF-CD74 axis augments radiotherapy efficacy for brain metastasis in NSCLC via synergistically promoting microglia M1 polarization
- PMID: 38685050
- PMCID: PMC11059744
- DOI: 10.1186/s13046-024-03024-9
Blocking the MIF-CD74 axis augments radiotherapy efficacy for brain metastasis in NSCLC via synergistically promoting microglia M1 polarization
Abstract
Background: Brain metastasis is one of the main causes of recurrence and death in non-small cell lung cancer (NSCLC). Although radiotherapy is the main local therapy for brain metastasis, it is inevitable that some cancer cells become resistant to radiation. Microglia, as macrophages colonized in the brain, play an important role in the tumor microenvironment. Radiotherapy could activate microglia to polarize into both the M1 and M2 phenotypes. Therefore, searching for crosstalk molecules within the microenvironment that can specifically regulate the polarization of microglia is a potential strategy for improving radiation resistance.
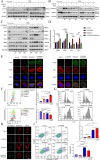
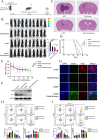
Methods: We used databases to detect the expression of MIF in NSCLC and its relationship with prognosis. We analyzed the effects of targeted blockade of the MIF/CD74 axis on the polarization and function of microglia during radiotherapy using flow cytometry. The mouse model of brain metastasis was used to assess the effect of targeted blockade of MIF/CD74 axis on the growth of brain metastasis.
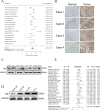
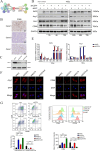
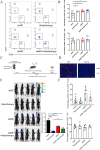
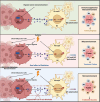
Result: Our findings reveals that the macrophage migration inhibitory factor (MIF) was highly expressed in NSCLC and is associated with the prognosis of NSCLC. Mechanistically, we demonstrated CD74 inhibition reversed radiation-induced AKT phosphorylation in microglia and promoted the M1 polarization in combination of radiation. Additionally, blocking the MIF-CD74 interaction between NSCLC and microglia promoted microglia M1 polarization. Furthermore, radiation improved tumor hypoxia to decrease HIF-1α dependent MIF secretion by NSCLC. MIF inhibition enhanced radiosensitivity for brain metastasis via synergistically promoting microglia M1 polarization in vivo.
Conclusions: Our study revealed that targeting the MIF-CD74 axis promoted microglia M1 polarization and synergized with radiotherapy for brain metastasis in NSCLC.
Keywords: Brain metastases; CD74; MIF; Microglia; NSCLC; Radiotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

