Liraglutide and not lifestyle intervention reduces soluble CD163 after comparable weight loss in obese participants with prediabetes or type 2 diabetes mellitus
- PMID: 38685051
- PMCID: PMC11059692
- DOI: 10.1186/s12933-024-02237-8
Liraglutide and not lifestyle intervention reduces soluble CD163 after comparable weight loss in obese participants with prediabetes or type 2 diabetes mellitus
Abstract
Background: The GLP-1 receptor agonist liraglutide is used to treat hyperglycemia in type 2 diabetes but is also known to induce weight loss, preserve the beta cell and reduce cardiovascular risk. The mechanisms underlying these effects are however still not completely known. Herein we explore the effect of liraglutide on markers of immune cell activity in a population of obese individuals with prediabetes or newly diagnosed type 2 diabetes mellitus.
Method: Plasma levels of the monocyte/macrophage markers, soluble (s)CD163 and sCD14, the neutrophil markers myeloperoxidase (MPO) and neutrophil gelatinase-associated lipocalin (NGAL),the T-cell markers sCD25 and T-cell immunoglobulin mucin domain-3 (sTIM-3) and the inflammatory marker TNF superfamily (TNFSF) member 14 (LIGHT/TNFSF14) were measured by enzyme-linked immunosorbent assays in obese individuals with prediabetes or diabetes diagnosed within the last 12 months, prior to and after comparable weight loss achieved with lifestyle changes (n = 20) or liraglutide treatment (n = 20), and in healthy subjects (n = 13).
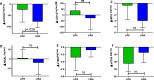
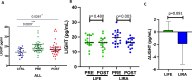
Results: At baseline, plasma levels of the macrophage marker sCD163, and the inflammatory marker LIGHT were higher in cases as compared to controls. Plasma levels of sCD14, NGAL, sTIM-3 and sCD25 did not differ at baseline between patients and controls. After weight reduction following lifestyle intervention or liraglutide treatment, sCD163 decreased significantly in the liraglutide group vs. lifestyle (between-group difference p = 0.023, adjusted for visceral adipose tissue and triglycerides basal values). MPO and LIGHT decreased significantly only in the liraglutide group (between group difference not significant). Plasma levels of MPO and in particular sCD163 correlated with markers of metabolic dysfunction and inflammation. After weight loss, only sCD163 showed a trend for decreased levels during OGTT, both in the whole cohort as in those of liraglutide vs lifestyle group.
Conclusion: Weight loss following treatment with liraglutide was associated with reduced circulating levels of sCD163 when compared to the same extent of weight loss after lifestyle changes. This might contribute to reduced cardiometabolic risk in individuals receiving treatment with liraglutide.
Keywords: GLP-1 analogue; Immune cells; Obesity; T2DM; Weight loss.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Interleukin-1β in circulating mononuclear cells predicts steatotic liver disease improvement after weight loss in subjects with obesity and prediabetes or type 2 diabetes.Cardiovasc Diabetol. 2025 Jun 13;24(1):247. doi: 10.1186/s12933-025-02706-8. Cardiovasc Diabetol. 2025. PMID: 40514652 Free PMC article. Clinical Trial.
-
Effects of liraglutide vs. lifestyle changes on soluble suppression of tumorigenesis-2 (sST2) and galectin-3 in obese subjects with prediabetes or type 2 diabetes after comparable weight loss.Cardiovasc Diabetol. 2022 Mar 11;21(1):36. doi: 10.1186/s12933-022-01469-w. Cardiovasc Diabetol. 2022. PMID: 35277168 Free PMC article. Clinical Trial.
-
Effects of Liraglutide on Weight Loss, Fat Distribution, and β-Cell Function in Obese Subjects With Prediabetes or Early Type 2 Diabetes.Diabetes Care. 2017 Nov;40(11):1556-1564. doi: 10.2337/dc17-0589. Epub 2017 Sep 14. Diabetes Care. 2017. PMID: 28912305 Clinical Trial.
-
Soluble CD163.Scand J Clin Lab Invest. 2012 Feb;72(1):1-13. doi: 10.3109/00365513.2011.626868. Epub 2011 Nov 7. Scand J Clin Lab Invest. 2012. PMID: 22060747 Review.
-
Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in the Treatment of Obese Women with Polycystic Ovary Syndrome.Curr Vasc Pharmacol. 2017;15(3):218-229. doi: 10.2174/1570161114666161221115324. Curr Vasc Pharmacol. 2017. PMID: 28003008 Review.
Cited by
-
Insulin resistance, Ca2+ signaling alterations and vascular dysfunction in prediabetes and metabolic syndrome.Front Physiol. 2025 Jun 10;16:1535153. doi: 10.3389/fphys.2025.1535153. eCollection 2025. Front Physiol. 2025. PMID: 40556956 Free PMC article. Review.
-
Interleukin-1β in circulating mononuclear cells predicts steatotic liver disease improvement after weight loss in subjects with obesity and prediabetes or type 2 diabetes.Cardiovasc Diabetol. 2025 Jun 13;24(1):247. doi: 10.1186/s12933-025-02706-8. Cardiovasc Diabetol. 2025. PMID: 40514652 Free PMC article. Clinical Trial.
-
Targeting oxidative damage in diabetic foot ulcers: integrative strategies involving antioxidant drugs and nanotechnologies.Burns Trauma. 2025 Mar 10;13:tkaf020. doi: 10.1093/burnst/tkaf020. eCollection 2025. Burns Trauma. 2025. PMID: 40718700 Free PMC article. Review.
-
The CD163/TWEAK/Fn14 axis: A potential therapeutic target for alleviating inflammatory bone loss.J Orthop Translat. 2024 Oct 4;49:82-95. doi: 10.1016/j.jot.2024.09.002. eCollection 2024 Nov. J Orthop Translat. 2024. PMID: 39430128 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

