Neuronal Panx1 drives peripheral sensitization in experimental plantar inflammatory pain
- PMID: 38685116
- PMCID: PMC11057180
- DOI: 10.1186/s40779-024-00525-8
Neuronal Panx1 drives peripheral sensitization in experimental plantar inflammatory pain
Abstract
Background: The channel-forming protein Pannexin1 (Panx1) has been implicated in both human studies and animal models of chronic pain, but the underlying mechanisms remain incompletely understood.
Methods: Wild-type (WT, n = 24), global Panx1 KO (n = 24), neuron-specific Panx1 KO (n = 20), and glia-specific Panx1 KO (n = 20) mice were used in this study at Albert Einstein College of Medicine. The von Frey test was used to quantify pain sensitivity in these mice following complete Freund's adjuvant (CFA) injection (7, 14, and 21 d). The qRT-PCR was employed to measure mRNA levels of Panx1, Panx2, Panx3, Cx43, Calhm1, and β-catenin. Laser scanning confocal microscopy imaging, Sholl analysis, and electrophysiology were utilized to evaluate the impact of Panx1 on neuronal excitability and morphology in Neuro2a and dorsal root ganglion neurons (DRGNs) in which Panx1 expression or function was manipulated. Ethidium bromide (EtBr) dye uptake assay and calcium imaging were employed to investigate the role of Panx1 in adenosine triphosphate (ATP) sensitivity. β-galactosidase (β-gal) staining was applied to determine the relative cellular expression levels of Panx1 in trigeminal ganglia (TG) and DRG of transgenic mice.
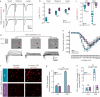
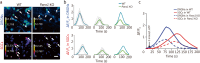
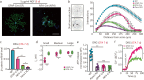
Results: Global or neuron-specific Panx1 deletion markedly decreased pain thresholds after CFA stimuli (7, 14, and 21 d; P < 0.01 vs. WT group), indicating that Panx1 was positively correlated with pain sensitivity. In Neuro2a, global Panx1 deletion dramatically reduced neurite extension and inward currents compared to the WT group (P < 0.05), revealing that Panx1 enhanced neurogenesis and excitability. Similarly, global Panx1 deletion significantly suppressed Wnt/β-catenin dependent DRG neurogenesis following 5 d of nerve growth factor (NGF) treatment (P < 0.01 vs. WT group). Moreover, Panx1 channels enhanced DRG neuron response to ATP after CFA injection (P < 0.01 vs. Panx1 KO group). Furthermore, ATP release increased Ca2+ responses in DRGNs and satellite glial cells surrounding them following 7 d of CFA treatment (P < 0.01 vs. Panx1 KO group), suggesting that Panx1 in glia also impacts exaggerated neuronal excitability. Interestingly, neuron-specific Panx1 deletion was found to markedly reduce differentiation in cultured DRGNs, as evidenced by stunted neurite outgrowth (P < 0.05 vs. Panx1 KO group; P < 0.01 vs. WT group or GFAP-Cre group), blunted activation of Wnt/β-catenin signaling (P < 0.01 vs. WT, Panx1 KO and GFAP-Cre groups), and diminished cell excitability (P < 0.01 vs. GFAP-Cre group) and response to ATP stimulation (P < 0.01 vs. WT group). Analysis of β-gal staining showed that cellular expression levels of Panx1 in neurons are significantly higher (2.5-fold increase) in the DRG than in the TG.
Conclusions: The present study revealed that neuronal Panx1 is a prominent driver of peripheral sensitivity in the setting of inflammatory pain through cell-autonomous effects on neuronal excitability. This hyperexcitability dependence on neuronal Panx1 contrasts with inflammatory orofacial pain, where similar studies revealed a prominent role for glial Panx1. The apparent differences in Panx1 expression in neuronal and non-neuronal TG and DRG cells are likely responsible for the distinct impact of these cell types in the two pain models.
Keywords: Dorsal root ganglion; Panx1; Peripheral sensitization; Plantar inflammatory pain; Satellite glial cell.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Comment in
-
The emerging role of Panx1 as a potential therapeutic target for chronic pain.Mil Med Res. 2024 Jul 5;11(1):44. doi: 10.1186/s40779-024-00549-0. Mil Med Res. 2024. PMID: 38970139 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

