Embolic strokes of undetermined source: a clinical consensus statement of the ESC Council on Stroke, the European Association of Cardiovascular Imaging and the European Heart Rhythm Association of the ESC
- PMID: 38685132
- PMCID: PMC11107123
- DOI: 10.1093/eurheartj/ehae150
Embolic strokes of undetermined source: a clinical consensus statement of the ESC Council on Stroke, the European Association of Cardiovascular Imaging and the European Heart Rhythm Association of the ESC
Abstract
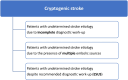
One in six ischaemic stroke patients has an embolic stroke of undetermined source (ESUS), defined as a stroke with unclear aetiology despite recommended diagnostic evaluation. The overall cardiovascular risk of ESUS is high and it is important to optimize strategies to prevent recurrent stroke and other cardiovascular events. The aim of clinicians when confronted with a patient not only with ESUS but also with any other medical condition of unclear aetiology is to identify the actual cause amongst a list of potential differential diagnoses, in order to optimize secondary prevention. However, specifically in ESUS, this may be challenging as multiple potential thromboembolic sources frequently coexist. Also, it can be delusively reassuring because despite the implementation of specific treatments for the individual pathology presumed to be the actual thromboembolic source, patients can still be vulnerable to stroke and other cardiovascular events caused by other pathologies already identified during the index diagnostic evaluation but whose thromboembolic potential was underestimated. Therefore, rather than trying to presume which particular mechanism is the actual embolic source in an ESUS patient, it is important to assess the overall thromboembolic risk of the patient through synthesis of the individual risks linked to all pathologies present, regardless if presumed causally associated or not. In this paper, a multi-disciplinary panel of clinicians/researchers from various backgrounds of expertise and specialties (cardiology, internal medicine, neurology, radiology and vascular surgery) proposes a comprehensive multi-dimensional assessment of the overall thromboembolic risk in ESUS patients through the composition of individual risks associated with all prevalent pathologies.
Keywords: Aetiology; Atherosclerosis; Cancer; Embolic stroke of undetermined source; Left atrial disease; Left ventricular disease; Patent foramen ovale; Valvular heart disease.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures