Machine learning in time-lapse imaging to differentiate embryos from young vs old mice†
- PMID: 38685607
- PMCID: PMC11180621
- DOI: 10.1093/biolre/ioae056
Machine learning in time-lapse imaging to differentiate embryos from young vs old mice†
Abstract
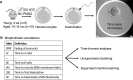
Time-lapse microscopy for embryos is a non-invasive technology used to characterize early embryo development. This study employs time-lapse microscopy and machine learning to elucidate changes in embryonic growth kinetics with maternal aging. We analyzed morphokinetic parameters of embryos from young and aged C57BL6/NJ mice via continuous imaging. Our findings show that aged embryos accelerated through cleavage stages (from 5-cells) to morula compared to younger counterparts, with no significant differences observed in later stages of blastulation. Unsupervised machine learning identified two distinct clusters comprising of embryos from aged or young donors. Moreover, in supervised learning, the extreme gradient boosting algorithm successfully predicted the age-related phenotype with 0.78 accuracy, 0.81 precision, and 0.83 recall following hyperparameter tuning. These results highlight two main scientific insights: maternal aging affects embryonic development pace, and artificial intelligence can differentiate between embryos from aged and young maternal mice by a non-invasive approach. Thus, machine learning can be used to identify morphokinetics phenotypes for further studies. This study has potential for future applications in selecting human embryos for embryo transfer, without or in complement with preimplantation genetic testing.
Keywords: machine learning; maternal aging; morphokinetics; predictive modeling; preimplantation mouse embryos; time-lapse microscopy.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures




References
-
- Meseguer M, Pellicer A. One for all or all for one? The evolution of embryo morphokinetics. Fertil Steril 2017; 107:571–572. - PubMed
-
- Jiang VS, Bormann CL. Noninvasive genetic screening: current advances in artificial intelligence for embryo ploidy prediction. Fertil Steril 2023; 120:228–234. - PubMed
-
- Storr A, Venetis C, Cooke S, Kilani S, Ledger W. Time-lapse algorithms and morphological selection of day-5 embryos for transfer: a preclinical validation study. Fertil Steril 2018; 109:276–283.e3. - PubMed
-
- Aparicio-Ruiz B, Basile N, Perez Albala S, Bronet F, Remohi J, Meseguer M. Automatic time-lapse instrument is superior to single-point morphology observation for selecting viable embryos: retrospective study in oocyte donation. Fertil Steril 2016; 106:1379–1385.e10. - PubMed
MeSH terms
Grants and funding
- Career Development Award
- UL1 TR001863/TR/NCATS NIH HHS/United States
- Office of Research and Development
- #CIN 13-407/Health Services Research and Development
- UM1 HG006348/NH/NIH HHS/United States
- IK2 CX001981/CX/CSRD VA/United States
- K12 HD047018/HD/NICHD NIH HHS/United States
- 5K12HD047018/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- Baylor College of Medicine Department of Obstetrics and Gynecology
- VA/VA/United States
- Department of Veterans Affairs, Veterans Health Administration
- UM1 HG006348/HG/NHGRI NIH HHS/United States

