Neoadjuvant Chemotherapy in Patients with HER2-Negative Breast Cancer: A Report from Clinical Breast Cancer Registry of Iran
- PMID: 38685847
- PMCID: PMC11097303
- DOI: 10.34172/aim.2024.30
Neoadjuvant Chemotherapy in Patients with HER2-Negative Breast Cancer: A Report from Clinical Breast Cancer Registry of Iran
Abstract
Background: Neoadjuvant chemotherapy (NCT) has become an increasingly popular approach in management of breast cancer (BC). This study was conducted to evaluate the pathologic response and 36-month recurrence and survival rates of patients with human epidermal growth factor receptor 2 (HER2)-negative BC treated with different NCT regimens.
Methods: A total of 163 female patients with HER2-negative BC who received NCT during 2017-2020 were identified from the Clinical Breast Cancer Registry of Iran and entered the study. The prescribed NCT regimens included 4 cycles of doxorubicin plus cyclophosphamide, 4 cycles of doxorubicin plus cyclophosphamide followed by 4 cycles of paclitaxel, 4 cycles of doxorubicin plus cyclophosphamide followed by 4 cycles of docetaxel or 6 cycles of doxorubicin plus cyclophosphamide plus docetaxel (TAC).
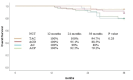
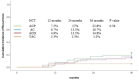
Results: Thirty-two patients (19.6%) experienced pathologic complete response (pCR). TAC regimen, triple negative-BC and ki67>10% were significantly associated with increased pCR. The recurrence, overall survival (OS) and disease-free survival (DFS) rate at 36 months for all patients were 16.6%, 84.7% and 79.8%, respectively. Type of neoadjuvant regimen as well as age, hormone receptor status, Ki67, grade, clinical stage, type of surgery and pathologic response to chemotherapy did not significantly influence the survival and recurrence; however, TAC results in improved recurrence, OS and DFS rates.
Conclusion: This study provides further evidence that NCT is a viable treatment option for patients with HER2-negative BC. The TAC regimen resulted in a significantly higher pCR rate compared to other regimens, but did not result in a significant improvement in recurrence, OS and DFS and rates.
Keywords: Breast cancer; HER2-negative; Pathologic complete response; Recurrence; Survival.
© 2024 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Conflict of interest statement
The authors declare no conflict of interest.
Similar articles
-
Proliferation Determined by Ki-67 Defines Different Pathologic Response to Neoadjuvant Trastuzumab-Based Chemotherapy in HER2-Positive Breast Cancer.Clin Breast Cancer. 2015 Oct;15(5):343-7. doi: 10.1016/j.clbc.2015.01.005. Epub 2015 Jan 21. Clin Breast Cancer. 2015. PMID: 25752727
-
Response and Prognosis of Docetaxel and Cyclophosphamide as Neoadjuvant Chemotherapy in ER+ HER2- Breast Cancer: A Prospective Phase II Study.Clin Breast Cancer. 2020 Dec;20(6):462-468. doi: 10.1016/j.clbc.2020.09.007. Epub 2020 Sep 18. Clin Breast Cancer. 2020. PMID: 33046356 Clinical Trial.
-
Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): secondary outcomes of a phase 3, randomised controlled trial.Lancet Oncol. 2015 Sep;16(9):1037-1048. doi: 10.1016/S1470-2045(15)00041-8. Epub 2015 Aug 10. Lancet Oncol. 2015. PMID: 26272770 Free PMC article. Clinical Trial.
-
Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer.Cochrane Database Syst Rev. 2019 Feb 18;2(2):CD012873. doi: 10.1002/14651858.CD012873.pub2. Cochrane Database Syst Rev. 2019. PMID: 30776132 Free PMC article.
-
Comparison of the Pathological Complete Response Rate and Survival Between HER2-Low and HER2-Zero Breast Cancer in Neoadjuvant Chemotherapy Setting: A Systematic Review and Meta-Analysis.Clin Breast Cancer. 2024 Oct;24(7):575-584.e1. doi: 10.1016/j.clbc.2024.05.001. Epub 2024 May 10. Clin Breast Cancer. 2024. PMID: 38821742
References
-
- Gonzalez L, Bardach A, Palacios A, Peckaitis C, Ciapponi A, Pichón-Riviere A, et al. Health-related quality of life in patients with breast cancer in Latin America and the Caribbean: a systematic review and meta-analysis. Oncologist. 2021;26(5):e794–806. doi: 10.1002/onco.13709. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous