5-chloro-3-(2-(2,4-dinitrophenyl) hydrazono)indolin-2-one: synthesis, characterization, biochemical and computational screening against SARS-CoV-2
- PMID: 38685970
- PMCID: PMC11055700
- DOI: 10.1007/s11696-023-03274-5
5-chloro-3-(2-(2,4-dinitrophenyl) hydrazono)indolin-2-one: synthesis, characterization, biochemical and computational screening against SARS-CoV-2
Abstract
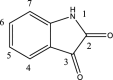
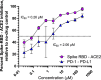
Chemical prototypes with broad-spectrum antiviral activity are important toward developing new therapies that can act on both existing and emerging viruses. Binding of the SARS-CoV-2 spike protein to the host angiotensin-converting enzyme 2 (ACE2) receptor is required for cellular entry of SARS-CoV-2. Toward identifying new chemical leads that can disrupt this interaction, including in the presence of SARS-CoV-2 adaptive mutations found in variants like omicron that can circumvent vaccine, immune, and therapeutic antibody responses, we synthesized 5-chloro-3-(2-(2,4-dinitrophenyl)hydrazono)indolin-2-one (H2L) from the condensation reaction of 5-chloroisatin and 2,4-dinitrophenylhydrazine in good yield. H2L was characterised by elemental and spectral (IR, electronic, Mass) analyses. The NMR spectrum of H2L indicated a keto-enol tautomerism, with the keto form being more abundant in solution. H2L was found to selectively interfere with binding of the SARS-CoV-2 spike receptor-binding domain (RBD) to the host angiotensin-converting enzyme 2 receptor with a 50% inhibitory concentration (IC50) of 0.26 μM, compared to an unrelated PD-1/PD-L1 ligand-receptor-binding pair with an IC50 of 2.06 μM in vitro (Selectivity index = 7.9). Molecular docking studies revealed that the synthesized ligand preferentially binds within the ACE2 receptor-binding site in a region distinct from where spike mutations in SARS-CoV-2 variants occur. Consistent with these models, H2L was able to disrupt ACE2 interactions with the RBDs from beta, delta, lambda, and omicron variants with similar activities. These studies indicate that H2L-derived compounds are potential inhibitors of multiple SARS-CoV-2 variants, including those capable of circumventing vaccine and immune responses.
Supplementary information: The online version contains supplementary material available at 10.1007/s11696-023-03274-5.
Keywords: Angiotensin-converting enzyme 2 receptor; Antivirals; Coronavirus; Isatin hydrazine; Molecular docking; SARS-CoV-2 spike.
© The Author(s) 2024.
Conflict of interest statement
Conflict of interestWe declare none.
Figures
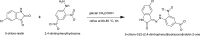

References
-
- Banks JL, Beard HS, Cao Y, Cho AE, Damm W, Farid R, Felts AK, Halgren TA, Mainz DT, Maple JR, Murphy R, Philipp DM, Repasky MP, Zhang LY, Berne BJ, Friesner RA, Gallicchio E, Levy RM. Integrated modeling program, applied chemical theory (IMPACT) J Comput Chem. 2005;26(16):1752–1780. doi: 10.1002/jcc.20292. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
