Evaluating first-line therapeutic strategies for metastatic castration-resistant prostate cancer: a comprehensive network meta-analysis and systematic review
- PMID: 38686197
- PMCID: PMC11056588
- DOI: 10.3389/fonc.2024.1378993
Evaluating first-line therapeutic strategies for metastatic castration-resistant prostate cancer: a comprehensive network meta-analysis and systematic review
Abstract
Objective: This study aimed to evaluate the relative efficacy and safety of first-line treatment options for metastatic castration-resistant prostate cancer (mCRPC).
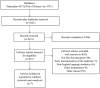
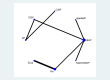
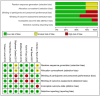
Methods: We systematically searched electronic databases, including PubMed and Web of Science, for studies published from their inception to April 3rd, 2023. Inclusion criteria were: 1) Completed Phase III or IV randomized controlled trials (RCTs) registered on ClinicalTrials.gov; 2) Patients with a confirmed diagnosis of mCRPC who had not previously received chemotherapy or novel endocrine therapies. We conducted a network meta-analysis using R software (version 3.4.0). Network graphs and risk of bias graphs were generated using Stata 14.0 and RevMan 5.4, respectively. The primary outcome was overall survival (OS), and the secondary outcome was the incidence of severe adverse events (SAEs).
Results: Seven RCTs encompassing 6,641 patients were included. The network meta-analysis revealed that both docetaxel+prednisone (DP) and cabazitaxel+prednisone (CP) significantly improved OS compared to abiraterone. Compared to placebo, DP showed comparable results to both cabazitaxel 20 mg/m^2+prednisone (C20P) and cabazitaxel 25 mg/m^2+prednisone (C25P) in terms of OS. For SAEs, both DP and C20P were superior to C25P, with no statistical difference between C20P and DP. The probability ranking plots indicated that C25P ranked highest for OS, while DP ranked highest for SAEs.
Conclusions: Based on our network meta-analysis, we recommend cabazitaxel 20 mg/m^2+prednisone (C20P) as the primary choice for first-line management of mCRPC, followed by DP. Enzalutamide and abiraterone are suggested as subsequent options. Radium-223 may be considered for patients presenting with bone metastases.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023443943.
Keywords: antihormone therapy; castration resistant prostate cancer; chemotherapy; first-line treatment; network meta-analysis.
Copyright © 2024 Zhang, Weng, Zhu, Gong and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Comparison of Systemic Treatments for Metastatic Castration-Resistant Prostate Cancer After Docetaxel Failure: A Systematic Review and Network Meta-analysis.Front Pharmacol. 2022 Jan 18;12:789319. doi: 10.3389/fphar.2021.789319. eCollection 2021. Front Pharmacol. 2022. PMID: 35115934 Free PMC article. Review.
-
The efficacy and safety comparison of docetaxel, cabazitaxel, estramustine, and mitoxantrone for castration-resistant prostate cancer: A network meta-analysis.Int J Surg. 2018 Aug;56:133-140. doi: 10.1016/j.ijsu.2018.06.010. Epub 2018 Jun 12. Int J Surg. 2018. PMID: 29906643
-
Efficacy and Safety of Cabazitaxel Versus Abiraterone or Enzalutamide in Older Patients with Metastatic Castration-resistant Prostate Cancer in the CARD Study.Eur Urol. 2021 Oct;80(4):497-506. doi: 10.1016/j.eururo.2021.06.021. Epub 2021 Jul 15. Eur Urol. 2021. PMID: 34274136 Clinical Trial.
-
Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial.Lancet Oncol. 2017 Nov;18(11):1532-1542. doi: 10.1016/S1470-2045(17)30605-8. Epub 2017 Oct 9. Lancet Oncol. 2017. PMID: 29033099 Clinical Trial.
-
Efficacy and safety of olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a systematic review and meta-analysis of randomized controlled trials.Front Oncol. 2023 Oct 6;13:1265276. doi: 10.3389/fonc.2023.1265276. eCollection 2023. Front Oncol. 2023. PMID: 37869079 Free PMC article.
Cited by
-
Safety and Efficacy of Reduced Dose of Enzalutamide in Patients with Castration-Resistant Prostate Cancer: A Systematic Review.Pharmaceuticals (Basel). 2025 May 16;18(5):732. doi: 10.3390/ph18050732. Pharmaceuticals (Basel). 2025. PMID: 40430550 Free PMC article. Review.
-
Modern Treatment of Skeletal Metastases: Multidisciplinarity and the Concept of Oligometastasis in the Recent Literature.Curr Oncol. 2025 Apr 11;32(4):226. doi: 10.3390/curroncol32040226. Curr Oncol. 2025. PMID: 40277781 Free PMC article. Review.
-
A Bayesian Network Meta-analysis of Systemic Treatments for Metastatic Castration-Resistant Prostate Cancer in First- and Subsequent Lines.Target Oncol. 2025 Jul;20(4):569-595. doi: 10.1007/s11523-025-01148-2. Epub 2025 Jun 10. Target Oncol. 2025. PMID: 40493311 Free PMC article.
References
-
- Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, et al. . Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. (2021) 398:131–42. doi: 10.1016/s0140-6736(21)00580-8 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

