Novel isatin-triazole based thiosemicarbazones as potential anticancer agents: synthesis, DFT and molecular docking studies
- PMID: 38686286
- PMCID: PMC11057040
- DOI: 10.1039/d4ra01937g
Novel isatin-triazole based thiosemicarbazones as potential anticancer agents: synthesis, DFT and molecular docking studies
Abstract
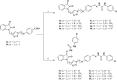
Thiosemicarbazones of isatin have been found to exhibit versatile bioactivities. In this study, two distinct types of isatin-triazole hybrids 3a and 3b were accessed via copper-catalyzed azide-alkyne cycloaddition reaction (CuAAC), together with their mono and bis-thiosemicarbazone derivatives 4a-h and 5a-h. In addition to the characterization by physical, spectral and analytical data, a DFT study was carried out to obtain the optimized geometries of all thiosemicarbazones. The global reactivity values showed that among the synthesized derivatives, 4c, 4g and 5c having nitro substituents are the most soft compounds, with compound 5c having the highest electronegativity and electrophilicity index values among the synthesized series, thus possessing strong binding ability with biomolecules. Molecular docking studies were performed to explore the inhibitory ability of the selected compounds against the active sites of the anticancer protein of phosphoinositide 3-kinase (PI3K). Among the synthesized derivatives, 4-nitro substituted bisthiosemicarbazone 5c showed the highest binding energy of -10.3 kcal mol-1. These findings demonstrated that compound 5c could be used as a favored anticancer scaffold via the mechanism of inhibition against the PI3K signaling pathways.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Mushtaq A. Wu P. Naseer M. M. Recent drug design strategies and identification of key heterocyclic scaffolds for promising anticancer targets. Pharmacol. Ther. 2024;254:108579. - PubMed
-
- Eftekhari-Sis B. Zirak M. Akbari A. Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev. 2013;113(5):2958–3043. - PubMed
-
- Wang Y. Liang Z. Zheng Y. Leung A. S.-L. Yan S.-C. So P.-K. Leung Y.-C. Wong W.-L. Wong K.-Y. Rational structural modification of the isatin scaffold to develop new and potent antimicrobial agents targeting bacterial peptidoglycan glycosyltransferase. RSC Adv. 2021;11(29):18122–18130. - PMC - PubMed
-
- Katiyar A. Hegde M. Kumar S. Gopalakrishnan V. Bhatelia K. D. Ananthaswamy K. Ramareddy S. A. De Clercq E. Choudhary B. Schols D. Synthesis and evaluation of the biological activity of N′-[2-oxo-1, 2 dihydro-3 H-indol-3-ylidene] benzohydrazides as potential anticancer agents. RSC Adv. 2015;5(56):45492–45501.
LinkOut - more resources
Full Text Sources
Miscellaneous

