Characterization and antitumor effect of doxorubicin-loaded Fe3O4-Au nanocomposite synthesized by electron beam evaporation for magnetic nanotheranostics
- PMID: 38686287
- PMCID: PMC11056945
- DOI: 10.1039/d4ra01777c
Characterization and antitumor effect of doxorubicin-loaded Fe3O4-Au nanocomposite synthesized by electron beam evaporation for magnetic nanotheranostics
Abstract
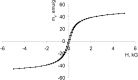
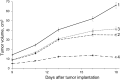
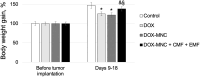
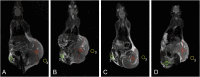
Magnetic nanocomposites (MNC) are promising theranostic platforms with tunable physicochemical properties allowing for remote drug delivery and multimodal imaging. Here, we developed doxorubicin-loaded Fe3O4-Au MNC (DOX-MNC) using electron beam physical vapor deposition (EB-PVD) in combination with magneto-mechanochemical synthesis to assess their antitumor effect on Walker-256 carcinosarcoma under the influence of a constant magnetic (CMF) and electromagnetic field (EMF) by comparing tumor growth kinetics, magnetic resonance imaging (MRI) scans and electron spin resonance (ESR) spectra. Transmission (TEM) and scanning electron microscopy (SEM) confirmed the formation of spherical magnetite nanoparticles with a discontinuous gold coating that did not significantly affect the ferromagnetic properties of MNC, as measured by vibrating-sample magnetometry (VSM). Tumor-bearing animals were divided into the control (no treatment), conventional doxorubicin (DOX), DOX-MNC and DOX-MNC + CMF + EMF groups. DOX-MNC + CMF + EMF resulted in 14% and 16% inhibition of tumor growth kinetics as compared with DOX and DOX-MNC, respectively. MRI visualization showed more substantial tumor necrotic changes after the combined treatment. Quantitative analysis of T2-weighted (T2W) images revealed the lowest value of skewness and a significant increase in tumor intensity in response to DOX-MNC + CMF + EMF as compared with the control (1.4 times), DOX (1.6 times) and DOX-MNC (1.8 times) groups. In addition, the lowest level of nitric oxide determined by ESR was found in DOX-MNC + CMF + EMF tumors, which was close to that of the muscle tissue in the contralateral limb. We propose that the reason for the relationship between the observed changes in MRI and ESR is the hyperfine interaction of nuclear and electron spins in mitochondria, as a source of free radical production. Therefore, these results point to the use of EB-PVD and magneto-mechanochemically synthesized Fe3O4-Au MNC loaded with DOX as a potential candidate for cancer magnetic nanotheranostic applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures







Similar articles
-
pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment.Pharmaceutics. 2023 Feb 26;15(3):771. doi: 10.3390/pharmaceutics15030771. Pharmaceutics. 2023. PMID: 36986632 Free PMC article.
-
Preparation and characterization of magnetic gold nanoparticles to be used as doxorubicin nanocarriers.Phys Med. 2014 Nov;30(7):843-8. doi: 10.1016/j.ejmp.2014.05.012. Epub 2014 Jun 18. Phys Med. 2014. PMID: 24950615
-
The comparison between superparamagnetic and ferromagnetic iron oxide nanoparticles for cancer nanotherapy in the magnetic resonance system.Nanotechnology. 2019 Oct 11;30(41):415701. doi: 10.1088/1361-6528/ab2ea7. Epub 2019 Jul 2. Nanotechnology. 2019. PMID: 31265997
-
Effects induced by a 50 Hz electromagnetic field and doxorubicin on Walker-256 carcinosarcoma growth and hepatic redox state in rats.Electromagn Biol Med. 2021 Oct 2;40(4):475-487. doi: 10.1080/15368378.2021.1958342. Epub 2021 Aug 16. Electromagn Biol Med. 2021. PMID: 34392747
-
Cell-Penetrating Peptidic GRP78 Ligand-Conjugated Iron Oxide Magnetic Nanoparticles for Tumor-Targeted Doxorubicin Delivery and Imaging.ACS Appl Bio Mater. 2023 Mar 20;6(3):1019-1031. doi: 10.1021/acsabm.2c00897. Epub 2023 Mar 2. ACS Appl Bio Mater. 2023. PMID: 36862384
References
-
- Gorobets S. V. Gorobets O. Yu. Kovalova S. O. Bioinformatic analysis of the genetic mechanism of biomineralization of biogenic magnetic nanoparticles in bacteria capable of tumor-specific accumulation. Innovative Biosyst. Bioeng. 2022;6:48–55. doi: 10.20535/ibb.2022.6.2.260183. - DOI
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

