The CNIC-polypill (acetylsalicylic acid, atorvastatin, and ramipril), an effective and cost-saving secondary prevention strategy compared with other therapeutic options in patients with ischaemic heart disease
- PMID: 38686352
- PMCID: PMC11056486
- DOI: 10.1093/ehjopen/oeae027
The CNIC-polypill (acetylsalicylic acid, atorvastatin, and ramipril), an effective and cost-saving secondary prevention strategy compared with other therapeutic options in patients with ischaemic heart disease
Abstract
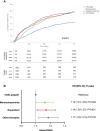
Aims: The retrospective NEPTUNO study evaluated the effectiveness of the Centro Nacional de Investigaciones Cardiovasculares (CNIC)-polypill (including acetylsalicylic acid, ramipril, and atorvastatin) vs. other therapeutic approaches in secondary prevention for cardiovascular (CV) disease. In this substudy, the focus was on the subgroup of patients with ischaemic heart disease (IHD).
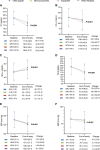
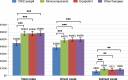
Methods and results: Patients on four strategies: CNIC-polypill, its monocomponents as loose medications, equipotent medications, and other therapies. The primary endpoint was the incidence of recurrent major adverse CV events (MACEs) after 2 years. After matching, 1080 patients were included in each cohort. The CNIC-polypill cohort had a significantly lower incidence of recurrent MACE compared with monocomponents, equipotent drugs, and other therapies cohorts (16.1 vs. 24, 24.4, and 24.3%, respectively; P < 0.001). The hazard ratios (HRs) for recurrent MACE were higher in monocomponents (HR = 1.12; P = 0.042), equipotent drugs (HR = 1.14; P = 0.031), and other therapies cohorts (HR = 1.17; P = 0.016) compared with the CNIC-polypill, with a number needed to treat of 12 patients to prevent a MACE. The CNIC-polypill demonstrated a greater reduction in LDL cholesterol (LDL-c; -56.1 vs. -43.6, -33.3, and -33.2% in the monocomponents, equipotent drugs, and other therapies, respectively; P < 0.001) and systolic blood pressure (-13.7 vs. -11.5, -10.6, and -9.1% in the CNIC-polypill, monocomponents, equipotent drugs, and other therapies, respectively; P < 0.001) compared with other cohorts. The CNIC-polypill intervention was less costly and more effective than any other therapeutic option, with €2317-€2407 cost savings per event prevented.
Conclusion: In IHD, the CNIC-polypill exemplifies a guideline-recommended secondary prevention treatment linked to better outcomes and cost saving compared with other therapeutic options.
Keywords: CNIC-polypill; Cardiovascular events; Healthcare costs; Ischaemic heart disease; MACE; Secondary prevention.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.D. reports consulting fees from Ferrer International, Amarin Sanofi, and Amgen; payment or honoraria for lectures from Novo Nordisk, Novartis, Ferrer, Amarin, Sanofi, Daiichi Sankio, and AMGEN; support for attending meetings and/or travel from Ferrer International, Novartis, Sanofi, Amarin, and Daiichi Sankio; has participated on Data Safety Monitoring Board or Advisory Board for Sanofi, and holds leadership or fiduciary roles in the World Heart Federation. A.C. reports honoraria consulting fees from AstraZeneca, Ferrer, Sanofi, AMGEN, Novartis, Lilly, Novo Nordisk, and Amarin; payment or honoraria for lectures from AstraZeneca, Ferrer, Sanofi, AMGEN, Novartis, Lilly, Novo Nordisk, and Amarin; and support for attending meetings and/or travel from Ferrer, Sanofi, AMGEN, Daiichi Sankio, Novartis, Novo Nordisk, and Amarin. L.M. reports honoraria consulting fees and payment or honoraria for lectures from Amgen, Sanofi, Novartis, Ferrer International, Servier, Daiichi Sankyo, Amarin, and Amryt. E.R. is a current worker at Ferrer International. A.S.-M. was working at Atrys Health at the time of the study. J.R.G.-J. reports honoraria for consulting and lectures from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, MSD, Daichi Sankyo, Ferrer International, Novartis, Lilly, Sanofi, and Servier.
Figures

Similar articles
-
The CNIC polypill (Acetylsalicylic acid + Atorvastatin + Rampril) in secondary prevention of cardiovascular disease in patients with type 2 diabetes: A comparative analysis with alternative therapeutic approaches.Int J Cardiol. 2025 Jan 1;418:132578. doi: 10.1016/j.ijcard.2024.132578. Epub 2024 Sep 19. Int J Cardiol. 2025. PMID: 39306297
-
Economic Burden Associated with the Treatment with a Cardiovascular Polypill in Secondary Prevention in Spain: Cost-Effectiveness Results of the NEPTUNO Study.Clinicoecon Outcomes Res. 2023 Jul 19;15:559-571. doi: 10.2147/CEOR.S396290. eCollection 2023. Clinicoecon Outcomes Res. 2023. PMID: 37489131 Free PMC article.
-
[Effectiveness of the CNIC polypill in secondary cardiovascular prevention in the subgroup of patients of the NEPTUNO study using atorvastatin doses of 20 mg].Arch Cardiol Mex. 2024 Jun 6;94(4):429-443. doi: 10.24875/ACM.23000230. Arch Cardiol Mex. 2024. PMID: 38843861 Free PMC article. Spanish.
-
The Fuster-CNIC-Ferrer Cardiovascular Polypill: a polypill for secondary cardiovascular prevention.Int J Cardiol. 2015 Dec;201 Suppl 1:S15-22. doi: 10.1016/S0167-5273(15)31028-7. Int J Cardiol. 2015. PMID: 26747390 Review.
-
Use of the cardiovascular polypill 40mg in secondary cardiovascular prevention.Clin Investig Arterioscler. 2018 Sep-Oct;30(5):240-247. doi: 10.1016/j.arteri.2018.04.004. Epub 2018 Jul 17. Clin Investig Arterioscler. 2018. PMID: 30017176 Review. English, Spanish.
Cited by
-
Intervention, improved prevention, imaging, inflammation, and innovation: the five I's cardiovascular highlights in EHJ Open 2024.Eur Heart J Open. 2025 Feb 28;5(1):oeaf015. doi: 10.1093/ehjopen/oeaf015. eCollection 2025 Jan. Eur Heart J Open. 2025. PMID: 40041611 Free PMC article. No abstract available.
-
Effectiveness and medication adherence in patients with ST- elevated myocardial infarction: Persian polypill study.ARYA Atheroscler. 2024;20(6):43-53. doi: 10.48305/arya.2025.43212.3007. ARYA Atheroscler. 2024. PMID: 40103623 Free PMC article.
References
-
- Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG; REACH Registry Investigators . Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–1357. - PubMed
-
- Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S; REACH Registry Investigators . One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197–1206. - PubMed
-
- Hess CN, Clare RM, Neely ML, Tricoci P, Mahaffey KW, James SK, Alexander JH, Held C, Lopes RD, Fox KAA, White HD, Wallentin L, Armstrong PW, Harrington RA, Ohman EM, Roe MT. Differential occurrence, profile, and impact of first recurrent cardiovascular events after an acute coronary syndrome. Am Heart J 2017;187:194–203. - PubMed
LinkOut - more resources
Full Text Sources

