Successful treatment with nivolumab in a patient with gastric cancer with severe liver failure resulting from multiple liver metastases: A case report
- PMID: 38686354
- PMCID: PMC11057026
- DOI: 10.3892/ol.2024.14404
Successful treatment with nivolumab in a patient with gastric cancer with severe liver failure resulting from multiple liver metastases: A case report
Abstract
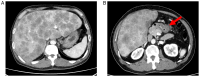
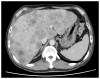
Gastric cancer (GC) is a globally prevalent and deadly malignancy often diagnosed at advanced stages, which can be accompanied by liver metastases. Conventional chemotherapy is contraindicated in patients with severe liver failure because several chemotherapeutic agents are metabolized by the liver. The present study reports on the successful use of nivolumab in a patient with advanced GC and severe liver failure owing to multiple liver metastases. A 57-year-old man was admitted to Shimane Prefectural Central Hospital (Izumo, Japan) with a 2-week history of appetite loss and jaundice. An upper gastrointestinal endoscopy revealed advanced GC (type IV). Computed tomography examination confirmed wall thickening of the gastric pylorus and the presence of multiple liver metastases. A gastric mucosal biopsy confirmed the diagnosis of HER2-positive gastric adenocarcinoma. S-1 + cisplatin chemotherapy was initiated but had to be halted due to the rapid deterioration in liver function, ultimately leading to acute liver failure. The patient was discharged from the hospital under palliative care. The patient was referred to Shimane University Hospital (Izumo, Japan) for a second consultation. Upon admission, the patient presented with severe liver failure, a Child-Pugh score of 10 (Class C), elevated total bilirubin levels of 13.9 mg/dl (normal range: <1.8 mg/dl) and elevated CEA and CA19-9. Nivolumab treatment was initiated, and notably, there was a substantial reduction in bilirubin levels, an improvement in liver function after a single cycle and a partial response observed in imaging studies. Despite the initial poor prognosis, the patient achieved long-term survival, ultimately succumbing to the illness 2 years and 6 months following the initiation of treatment. The present case underscores the potential of immune checkpoint inhibitors, such as nivolumab, in the treatment of patients with cancer and severe liver failure. It also challenges the conventional constraints of chemotherapy, offering a promising direction for future research in this area.
Keywords: GC; immune checkpoint inhibitors; multiple liver metastases; nivolumab; severe liver failure.
Copyright: © 2024 Ikejiri et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Successful R0 resection after chemotherapy, including nivolumab, for gastric cancer with liver metastases: three case reports.Surg Case Rep. 2024 Jun 5;10(1):138. doi: 10.1186/s40792-024-01929-3. Surg Case Rep. 2024. PMID: 38837046 Free PMC article.
-
Pathological complete response with nivolumab for recurrence of liver metastasis after gastrectomy of gastric cancer.Surg Case Rep. 2023 May 19;9(1):86. doi: 10.1186/s40792-023-01668-x. Surg Case Rep. 2023. PMID: 37204618 Free PMC article.
-
Successful treatment of liver metastases arising from early gastric cancer achieved clinical complete response by nivolumab.Surg Case Rep. 2018 Jul 5;4(1):71. doi: 10.1186/s40792-018-0479-3. Surg Case Rep. 2018. PMID: 29978335 Free PMC article.
-
Liver metastases from gastric carcinoma: A Case report and review of the literature.Curr Probl Cancer. 2017 May-Jun;41(3):222-230. doi: 10.1016/j.currproblcancer.2017.03.003. Epub 2017 Mar 24. Curr Probl Cancer. 2017. PMID: 28625333 Review.
-
Palliative Chemotherapy: Does It Only Provide False Hope? The Role of Palliative Care in a Young Patient With Newly Diagnosed Metastatic Adenocarcinoma.J Adv Pract Oncol. 2017 May-Jun;8(4):382-386. Epub 2017 May 1. J Adv Pract Oncol. 2017. PMID: 30018843 Free PMC article. Review.
References
-
- Wang L, Liang B, Jiang Y, Huang G, Tang A, Liu Z, Wang Y, Zhou R, Yang N, Wu J, et al. Subsite-specific metastatic organotropism and risk in gastric cancer: A population-based cohort study of the US SEER database and a Chinese single-institutional registry. Cancer Med. 2023;12:19595–19606. doi: 10.1002/cam4.6583. - DOI - PMC - PubMed
-
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. - DOI - PubMed
-
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
