Atherosclerosis Is a Smooth Muscle Cell-Driven Tumor-Like Disease
- PMID: 38686559
- PMCID: PMC11164647
- DOI: 10.1161/CIRCULATIONAHA.123.067587
Atherosclerosis Is a Smooth Muscle Cell-Driven Tumor-Like Disease
Abstract
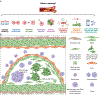
Background: Atherosclerosis, a leading cause of cardiovascular disease, involves the pathological activation of various cell types, including immunocytes (eg, macrophages and T cells), smooth muscle cells (SMCs), and endothelial cells. Accumulating evidence suggests that transition of SMCs to other cell types, known as phenotypic switching, plays a central role in atherosclerosis development and complications. However, the characteristics of SMC-derived cells and the underlying mechanisms of SMC transition in disease pathogenesis remain poorly understood. Our objective is to characterize tumor cell-like behaviors of SMC-derived cells in atherosclerosis, with the ultimate goal of developing interventions targeting SMC transition for the prevention and treatment of atherosclerosis.
Methods: We used SMC lineage tracing mice and human tissues and applied a range of methods, including molecular, cellular, histological, computational, human genetics, and pharmacological approaches, to investigate the features of SMC-derived cells in atherosclerosis.
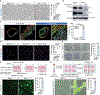
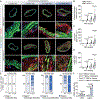
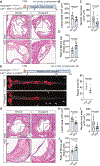
Results: SMC-derived cells in mouse and human atherosclerosis exhibit multiple tumor cell-like characteristics, including genomic instability, evasion of senescence, hyperproliferation, resistance to cell death, invasiveness, and activation of comprehensive cancer-associated gene regulatory networks. Specific expression of the oncogenic mutant KrasG12D in SMCs accelerates phenotypic switching and exacerbates atherosclerosis. Furthermore, we provide proof of concept that niraparib, an anticancer drug targeting DNA damage repair, attenuates atherosclerosis progression and induces regression of lesions in advanced disease in mouse models.
Conclusions: Our findings demonstrate that atherosclerosis is an SMC-driven tumor-like disease, advancing our understanding of its pathogenesis and opening prospects for innovative precision molecular strategies aimed at preventing and treating atherosclerotic cardiovascular disease.
Keywords: atherosclerosis; cardiovascular disease; smooth muscle cell.
Conflict of interest statement
Figures


Update of
-
Atherosclerosis is a smooth muscle cell-driven tumor-like disease.bioRxiv [Preprint]. 2023 Mar 7:2023.03.06.531330. doi: 10.1101/2023.03.06.531330. bioRxiv. 2023. Update in: Circulation. 2024 Jun 11;149(24):1885-1898. doi: 10.1161/CIRCULATIONAHA.123.067587. PMID: 36945644 Free PMC article. Updated. Preprint.
Comment in
-
A Concept of "Athero-Oncology": Tumor-Like Smooth Muscle Cells Drive Atherosclerosis.Circulation. 2024 Jun 11;149(24):1899-1902. doi: 10.1161/CIRCULATIONAHA.124.069446. Epub 2024 Jun 10. Circulation. 2024. PMID: 38857330 Free PMC article. No abstract available.
References
-
- Herrington W, Lacey B, Sherliker P, Armitage J and Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016;118:535–46. - PubMed
-
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr., Rosenfeld ME, Schwartz CJ, Wagner WD and Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–31. - PubMed
-
- Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR and Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727–744. - PubMed
-
- Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, Fong R, Woo YJ, Liu B, Montgomery SB, Wu JC, Zhu K, Chang R, Alamprese M, Tallquist MD, Kim JB and Quertermous T. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25:1280–1289. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

