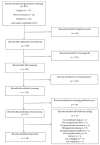
Exposure Assessment and Monitoring of Antiblastic Drugs Preparation in Health Care Settings: A Systematic Review
- PMID: 38686575
- PMCID: PMC11181217
- DOI: 10.23749/mdl.v115i2.15609
Exposure Assessment and Monitoring of Antiblastic Drugs Preparation in Health Care Settings: A Systematic Review
Abstract
Several antiblastic drugs (ADs) are classified as carcinogenic, mutagenic, and/or toxic for reproduction. Despite established guidelines and safe handling technologies, ADs contamination of the work environments could occur in healthcare settings, leading to potential exposure of healthcare staff. This systematic review aims to investigate the main techniques and practices for assessing ADs occupational exposure in healthcare settings. The reviewed studies unveil that workplace contamination by ADs appears to be a still-topical problem in healthcare settings. These issues are linked to difficulties in guaranteeing: (i) the adherence to standardized protocols when dealing with ADs, (ii) the effective use of personal protective equipment by operators involved in the administration or management of ADs, (iii) a comprehensive training of the healthcare personnel, and (iv) a thorough health surveillance of exposed workers. A "multi-parametric" approach emerges as a desirable strategy for exposure assessment. In parallel, exposure assessment should coincide with the introduction of novel technologies aimed at minimizing exposure (i.e., risk management). Assessment must consider various departments and health operators susceptible to ADs contamination, with a focus extended beyond worst-case scenarios, also considering activities like surface cleaning and logistical tasks related to ADs management. A comprehensive approach in ADs risk assessment enables the evaluation of distinct substance behaviors and subsequent exposure routes, affording a more holistic understanding of potential risks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jana S, Chakraborty D. Chemical Risk in Hospitals: An Overview of Monitoring Strategies and International Regulatory Practical Concerns. International Journal of Innovative Research in Engineering. 2023:562–70.
-
- European Commission. Second Edition. Luxembourg: Publications Office of the European Union; 2023. Guidance for the safe management of hazardous medicinal products at work.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical