Endogenous Protein-Protein Interaction Network of the NPC Cholesterol Transporter 1 in the Cerebral Cortex
- PMID: 38686625
- PMCID: PMC12131329
- DOI: 10.1021/acs.jproteome.3c00788
Endogenous Protein-Protein Interaction Network of the NPC Cholesterol Transporter 1 in the Cerebral Cortex
Abstract
NPC intracellular cholesterol transporter 1 (NPC1) is a multipass, transmembrane glycoprotein mostly recognized for its key role in facilitating cholesterol efflux. Mutations in the NPC1 gene result in Niemann-Pick disease, type C (NPC), a fatal, lysosomal storage disease. Due to the progressively expanding implications of NPC1-related disorders, we investigated endogenous NPC1 protein-protein interactions in the mouse cortex and human-derived iPSCs neuronal models of the disease through coimmunoprecipitation-coupled with LC-MS based proteomics. The current study investigated protein-protein interactions specific to the wild-type and the most prevalent NPC1 mutation (NPC1I1061T) while filtering out any protein interactor identified in the Npc1-/- mouse model. Additionally, the results were matched across the two species to map the parallel interactome of wild-type and mutant NPC1I1061T. Most of the identified wild-type NPC1 interactors were related to cytoskeleton organization, synaptic vesicle activity, and translation. We found many putative NPC1 interactors not previously reported, including two SCAR/WAVE complex proteins that regulate ARP 2/3 complex actin nucleation and multiple membrane proteins important for neuronal activity at synapse. Moreover, we identified proteins important in trafficking specific to wild-type and mutant NPC1I1061T. Together, the findings are essential for a comprehensive understanding of NPC1 biological functions in addition to its classical role in sterol efflux.
Keywords: Interactome; Lysosomal Storage Disorder; Neurodegenerative Disease; Niemann-Pick type C; Transmembrane Protein.
Conflict of interest statement
SMC, MSL, and APL serve as scientific advisors to SOAR-NPC. The funders had no role in the experimental design, data analysis or reporting of this work.
Figures

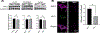
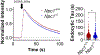

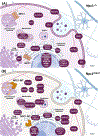
References
-
- Naureckiene S, et al., Identification of HE1 as the second gene of Niemann-Pick C disease. Science, 2000. 290(5500): p. 2298–301. - PubMed
-
- Neufeld EB, et al., The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem, 1999. 274(14): p. 9627–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

