DFMO inhibition of neuroblastoma tumorigenesis
- PMID: 38686627
- PMCID: PMC11058673
- DOI: 10.1002/cam4.7207
DFMO inhibition of neuroblastoma tumorigenesis
Abstract
Background: Most high-risk neuroblastoma patients who relapse succumb to disease despite the existing therapy. We recently reported increased event-free and overall survival in neuroblastoma patients receiving difluoromethylornithine (DFMO) during maintenance therapy. The effect of DFMO on cellular processes associated with neuroblastoma tumorigenesis needs further elucidation. Previous studies have shown cytotoxicity with IC50 values >5-15 mM, these doses are physiologically unattainable in patients, prompting further mechanistic studies at therapeutic doses.
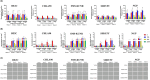
Methods: We characterized the effect of DFMO on cell viability, cell cycle, apoptosis, neurosphere formation, and protein expression in vitro using five established neuroblastoma cell lines (BE2C, CHLA-90, SHSY5Y, SMS-KCNR, and NGP) at clinically relevant doses of 0, 50, 100, 500, 1000, and 2500 μM. Limiting Dilution studies of tumor formation in murine models were performed. Statistical analysis was done using GraphPad and the level of significance set at p = 0.05.
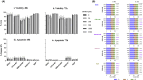
Results: There was not a significant loss of cell viability or gain of apoptotic activity in the in vitro assays (p > 0.05). DFMO treatment initiated G1 to S phase cell cycle arrest. There was a dose-dependent decrease in frequency and size of neurospheres and a dose-dependent increase in beta-galactosidase activity in all cell lines. Tumor formation was decreased in xenografts both with DFMO-pretreated cells and in mice treated with DFMO.
Conclusion: DFMO treatment is cytostatic at physiologically relevant doses and inhibits tumor initiation and progression in mice. This study suggests that DFMO, inhibits neuroblastoma by targeting cellular processes integral to neuroblastoma tumorigenesis at clinically relevant doses.
Keywords: DFMO; ELDA; cell cycle; neuroblastoma; neurosphere; senescence; xenograft.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All data included in this manuscript are freely available by contacting the corresponding author. All authors declare no potential conflict of interest.
Figures




References
-
- Neuroblastoma Survival Rates | American Cancer Society [Internet] . Accessed December 7, 2021. https://www.cancer.org/cancer/neuroblastoma/detection‐diagnosis‐staging/...
-
- Swift CC, Eklund MJ, Kraveka JM, Alazraki AL. Updates in diagnosis, management, and treatment of neuroblastoma. Radiographics. 2018;38:566‐580. - PubMed
-
- Tsubota S, Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. 2018;372:211‐221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

