Digital wearable insole-based identification of knee arthropathies and gait signatures using machine learning
- PMID: 38686919
- PMCID: PMC11152572
- DOI: 10.7554/eLife.86132
Digital wearable insole-based identification of knee arthropathies and gait signatures using machine learning
Abstract
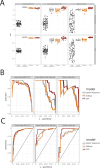
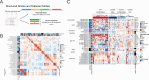
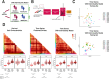
Gait is impaired in musculoskeletal conditions, such as knee arthropathy. Gait analysis is used in clinical practice to inform diagnosis and monitor disease progression or intervention response. However, clinical gait analysis relies on subjective visual observation of walking as objective gait analysis has not been possible within clinical settings due to the expensive equipment, large-scale facilities, and highly trained staff required. Relatively low-cost wearable digital insoles may offer a solution to these challenges. In this work, we demonstrate how a digital insole measuring osteoarthritis-specific gait signatures yields similar results to the clinical gait-lab standard. To achieve this, we constructed a machine learning model, trained on force plate data collected in participants with knee arthropathy and controls. This model was highly predictive of force plate data from a validation set (area under the receiver operating characteristics curve [auROC] = 0.86; area under the precision-recall curve [auPR] = 0.90) and of a separate, independent digital insole dataset containing control and knee osteoarthritis subjects (auROC = 0.83; auPR = 0.86). After showing that digital insole-derived gait characteristics are comparable to traditional gait measurements, we next showed that a single stride of raw sensor time-series data could be accurately assigned to each subject, highlighting that individuals using digital insoles can be identified by their gait characteristics. This work provides a framework for a promising alternative to traditional clinical gait analysis methods, adds to the growing body of knowledge regarding wearable technology analytical pipelines, and supports clinical development of at-home gait assessments, with the potential to improve the ease, frequency, and depth of patient monitoring.
Keywords: clinical AI/ML; data analysis; digital biomarkers; digital health technology; digital medicine; gait; human; medicine; neuroscience; wearables.
Plain language summary
The way we walk – our ‘gait’ – is a key indicator of health. Gait irregularities like limping, shuffling or a slow pace can be signs of muscle or joint problems. Assessing a patient’s gait is therefore an important element in diagnosing these conditions, and in evaluating whether treatments are working. Gait is often assessed via a simple visual inspection, with patients being asked to walk back and forth in a doctor’s office. While quick and easy, this approach is highly subjective and therefore imprecise. ‘Objective gait analysis’ is a more accurate alternative, but it relies on tests being conducted in specialised laboratories with large-scale, expensive equipment operated by highly trained staff. Unfortunately, this means that gait laboratories are not accessible for everyday clinical use. In response, Wipperman et al. aimed to develop a low-cost alternative to the complex equipment used in gait laboratories. To do this, they harnessed wearable sensor technologies – devices that can directly measure physiological data while embedded in clothing or attached to the user. Wearable sensors have the advantage of being cheap, easy to use, and able to provide clinically useful information without specially trained staff. Wipperman et al. analysed data from classic gait laboratory devices, as well as ‘digital insoles’ equipped with sensors that captured foot movements and pressure as participants walked. The analysis first ‘trained’ on data from gait laboratories (called force plates) and then applied the method to gait measurements obtained from digital insoles worn by either healthy participants or patients with knee problems. Analysis of the pressure data from the insoles confirmed that they could accurately predict which measurements were from healthy individuals, and which were from patients. The gait characteristics detected by the insoles were also comparable to lab-based measurements – in other words, the insoles provided similar type and quality of data as a gait laboratory. Further analysis revealed that information from just a single step could reveal additional information about the subject’s walking. These results support the use of wearable devices as a simple and relatively inexpensive way to measure gait in everyday clinical practice, without the need for specialised laboratories and visits to the doctor’s office. Although the digital insoles will require further analytical and clinical study before they can be widely used, Wipperman et al. hope they will eventually make monitoring muscle and joint conditions easier and more affordable.
© 2024, Wipperman, Lin, Gayvert et al.
Conflict of interest statement
MW, AL, KG, BL, SS, XW, JI, ML, BK, IS, MT, DD, AB, FW, WL, GH, JU, YP, GA, JH, SS, OL, AA, RA, SH, OH Employee and shareholder of Regeneron Pharmaceuticals, Inc
Figures












Update of
- doi: 10.1101/2022.10.05.22280750
References
-
- Alias M. Walking activity recognition with sEMG sensor array on thigh circumference using Convolutional neural network. International Conference 544 on Intelligent Systems Engineering (ICISE) IEEE.2018.
-
- Arslan YZ, Karabulut D, Ortes F, Popovic MB. In: Biomechatronics. Popovic MB, editor. Academic Press; 2019. 11 - Exoskeletons, Exomusculatures, Exosuits: dynamic modeling and simulation. - DOI
-
- Briouza S, Gritli H, Khraief N, Belghith S, Singh D. A Convolutional Neural Network-Based Architecture for EMG Signal Classification. 2021 International Conference on Data Analytics for Business and Industry (ICDABI; Sakheer, Bahrain. 2021. pp. 107–112. - DOI
-
- Celik Y, Stuart S, Woo WL, Sejdic E, Godfrey A. Multi-modal gait: a wearable, algorithm and data fusion approach for clinical and free-living assessment. Information Fusion. 2022;78:57–70. doi: 10.1016/j.inffus.2021.09.016. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

