Clinical and neuroimaging characteristics of primary lateral sclerosis with overlapping features of progressive supranuclear palsy
- PMID: 38686979
- PMCID: PMC11227385
- DOI: 10.1111/ene.16320
Clinical and neuroimaging characteristics of primary lateral sclerosis with overlapping features of progressive supranuclear palsy
Abstract
Background and purpose: Primary lateral sclerosis (PLS) is a neurodegenerative disorder that primarily affects the central motor system. In rare cases, clinical features of PLS may overlap with those of progressive supranuclear palsy (PSP). We investigate neuroimaging features that can help distinguish PLS with overlapping features of PSP (PLS-PSP) from PSP.
Methods: Six patients with PLS-PSP were enrolled between 2019 and 2023. We compared their clinical and neuroimaging characteristics with 18 PSP-Richardson syndrome (PSP-RS) patients and 20 healthy controls. Magnetic resonance imaging, 18F-flortaucipir positron emission tomography (PET), quantitative susceptibility mapping, and diffusion tensor imaging tractography (DTI) were performed to evaluate eight brain regions of interest. Area under the receiver operating characteristic curve (AUROC) was calculated.
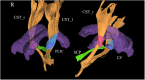
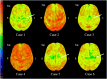
Results: Five of the six PLS-PSP patients (83.3%) were male. Median age at symptom onset was 61.5 (52.5-63) years, and all had mixed features of PLS and PSP. Volumes of the pallidum, caudate, midbrain, and cerebellar dentate were smaller in PSP-RS than PLS-PSP, providing good discrimination (AUROC = 0.75 for all). The susceptibilities in pallidum, midbrain, and cerebellar dentate were greater in PSP-RS compared to PLS-PSP, providing excellent discrimination (AUROC ≥ 0.90 for all). On DTI, fractional anisotropy (FA) in the posterior limb of the internal capsule from the corticospinal tract was lower in PLS-PSP compared to PSP-RS (AUROC = 0.86), but FA in the superior cerebellar peduncle was lower in PSP-RS (AUROC = 0.95). Pallidum flortaucipir PET uptake was greater in PSP-RS compared to PLS-PSP (AUROC = 0.74).
Conclusions: Regional brain volume, tractography, and magnetic susceptibility, but not tau-PET, are useful in distinguishing PLS-PSP from PSP.
Keywords: neurodegenerative disorders; primary lateral sclerosis; progressive supranuclear palsy; quantitative susceptibility mapping; tractography.
© 2024 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Multimodal neuroimaging relationships in progressive supranuclear palsy.Parkinsonism Relat Disord. 2019 Sep;66:56-61. doi: 10.1016/j.parkreldis.2019.07.001. Epub 2019 Jul 2. Parkinsonism Relat Disord. 2019. PMID: 31279635 Free PMC article.
-
Cerebral Tau Deposition in Comorbid Progressive Supranuclear Palsy and Amyotrophic Lateral Sclerosis: An [18F]-Flortaucipir and 7T MRI Study.Neurodegener Dis. 2023;23(3-4):35-42. doi: 10.1159/000536614. Epub 2024 Mar 25. Neurodegener Dis. 2023. PMID: 38527450 Free PMC article.
-
Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants.Neuroimage Clin. 2020;25:102152. doi: 10.1016/j.nicl.2019.102152. Epub 2019 Dec 28. Neuroimage Clin. 2020. PMID: 31935638 Free PMC article.
-
Diffusion Tensor Imaging in Progressive Supranuclear Palsy Versus Other Neurodegenerative Diseases: A Review.J Neuroimaging. 2025 May-Jun;35(3):e70063. doi: 10.1111/jon.70063. J Neuroimaging. 2025. PMID: 40524370 Review.
-
Differentiation of progressive supranuclear palsy: clinical, imaging and laboratory tools.Acta Neurol Scand. 2013 May;127(5):362-70. doi: 10.1111/ane.12067. Epub 2013 Feb 13. Acta Neurol Scand. 2013. PMID: 23406296 Review.
Cited by
-
Diffusion tensor imaging along the perivascular space: the bias from crossing fibres.Brain Commun. 2024 Nov 21;6(6):fcae421. doi: 10.1093/braincomms/fcae421. eCollection 2024. Brain Commun. 2024. PMID: 39713238 Free PMC article.
-
Patterns of glucose hypometabolism can help differentiate FTLD-FET from other types of FTLD.J Neurol. 2024 Sep;271(9):6264-6273. doi: 10.1007/s00415-024-12583-y. Epub 2024 Aug 1. J Neurol. 2024. PMID: 39088063 Free PMC article.
-
Looking into Abnormal Co-Expressions of Tau and TDP-43 in the Realm of Mixed Dementia Types: A Double-Punch Scenario.Brain Sci. 2025 Jul 3;15(7):716. doi: 10.3390/brainsci15070716. Brain Sci. 2025. PMID: 40722308 Free PMC article. Review.
-
Brainstem and cerebellar radiological findings in progressive supranuclear palsy.Brain Commun. 2025 Feb 5;7(1):fcaf051. doi: 10.1093/braincomms/fcaf051. eCollection 2025. Brain Commun. 2025. PMID: 39958262 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

