Broadly neutralizing antibodies for HIV prevention: a comprehensive review and future perspectives
- PMID: 38687039
- PMCID: PMC11324036
- DOI: 10.1128/cmr.00152-22
Broadly neutralizing antibodies for HIV prevention: a comprehensive review and future perspectives
Abstract
SUMMARYThe human immunodeficiency virus (HIV) epidemic remains a formidable global health concern, with 39 million people living with the virus and 1.3 million new infections reported in 2022. Despite anti-retroviral therapy's effectiveness in pre-exposure prophylaxis, its global adoption is limited. Broadly neutralizing antibodies (bNAbs) offer an alternative strategy for HIV prevention through passive immunization. Historically, passive immunization has been efficacious in the treatment of various diseases ranging from oncology to infectious diseases. Early clinical trials suggest bNAbs are safe, tolerable, and capable of reducing HIV RNA levels. Although challenges such as bNAb resistance have been noted in phase I trials, ongoing research aims to assess the additive or synergistic benefits of combining multiple bNAbs. Researchers are exploring bispecific and trispecific antibodies, and fragment crystallizable region modifications to augment antibody efficacy and half-life. Moreover, the potential of other antibody isotypes like IgG3 and IgA is under investigation. While promising, the application of bNAbs faces economic and logistical barriers. High manufacturing costs, particularly in resource-limited settings, and logistical challenges like cold-chain requirements pose obstacles. Preliminary studies suggest cost-effectiveness, although this is contingent on various factors like efficacy and distribution. Technological advancements and strategic partnerships may mitigate some challenges, but issues like molecular aggregation remain. The World Health Organization has provided preferred product characteristics for bNAbs, focusing on optimizing their efficacy, safety, and accessibility. The integration of bNAbs in HIV prophylaxis necessitates a multi-faceted approach, considering economic, logistical, and scientific variables. This review comprehensively covers the historical context, current advancements, and future avenues of bNAbs in HIV prevention.
Keywords: HIV; broadly neutralizing antibodies; monoclonal antibodies; prevention.
Conflict of interest statement
The author declares no conflict of interest.
Figures
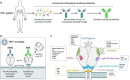


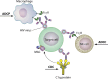

References
-
- UNAIDS . Global HIV & AIDS statistics — fact sheet. Available from: https://www.unaids.org/en/resources/fact-sheet
-
- Pegu A, Borate B, Huang Y, Pauthner MG, Hessell AJ, Julg B, Doria-Rose NA, Schmidt SD, Carpp LN, Cully MD, Chen X, Shaw GM, Barouch DH, Haigwood NL, Corey L, Burton DR, Roederer M, Gilbert PB, Mascola JR, Huang Y. 2019. A meta-analysis of passive immunization studies shows that serum-neutralizing antibody titer associates with protection against SHIV challenge. Cell Host Microbe 26:336–346. doi: 10.1016/j.chom.2019.08.014 - DOI - PMC - PubMed
-
- GBD 2017 HIV collaborators . 2019. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 6:e831–e859. doi: 10.1016/S2352-3018(19)30196-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

