Antimicrobial activity of ceftazidime-avibactam against KPC-2-producing Enterobacterales: a cross-combination and dose-escalation titration study with relebactam and vaborbactam
- PMID: 38687076
- PMCID: PMC11237450
- DOI: 10.1128/spectrum.00344-24
Antimicrobial activity of ceftazidime-avibactam against KPC-2-producing Enterobacterales: a cross-combination and dose-escalation titration study with relebactam and vaborbactam
Abstract
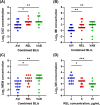
With the introduction of ceftazidime-avibactam worldwide, the antimicrobial activity of new β-lactam/β-lactamase inhibitors (BL/BLIs) needs to be investigated. From January 2020 to June 2023, Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacterales were collected. With a broth microdilution test of new BL/BLIs, cross-activity test with nine combinations of BLs and new BLIs and dose-escalation titration test for non-susceptible isolates were conducted to investigate inhibitory activities of new BLIs. A total of 188 isolates was collected and most isolates (186/188, 98.9%) carried the KPC-2 gene exclusively, while two isolates (1.1%) co-harbored NDM-1. Among the 186 KPC-2-producing isolates, 184 (98.9%) were susceptible to ceftazidime-avibactam, 173 (93.0%) to imipenem-relebactam, and 184 (98.9%) to meropenem-vaborbactam. All isolates non-susceptible to imipenem-relebactam or meropenem-vaborbactam became susceptible when avibactam replaced relebactam or vaborbactam, with 7 of 11 (63.6%) imipenem-relebactam non-susceptible isolates and both (100.0%) of the meropenem-vaborbactam non-susceptible isolates. When the minimum inhibitory concentrations (MICs) of BLs were compared using log2 scales, combinations with avibactam showed statistically significant efficacy in lowering MICs compared to relebactam and vaborbactam (all P < 0.05). In the dose-escalation test of new BLIs, increasing dose of all new BLIs corresponded to increased susceptibility to BLs. Ceftazidime-avibactam exhibited excellent susceptibility against KPC-2-producing Enterobacterales unless co-harboring metallo-β-lactamase. The cross-combination test against non-susceptible isolates suggests that the inhibitory activity of avibactam was superior to those of relebactam or vaborbactam. Increasing the dose of new BLIs produced increased susceptibility to BLs, suggesting that high-concentration regimen need to be developed.
Importance: This study investigated 188 Klebsiella pneumoniae carbapenemase (KPC)-2-producing Enterobacterales collected from January 2020 to June 2023 in a tertiary care hospital of Korea. Most isolates were susceptible to ceftazidime-avibactam (98.9%) and meropenem-vaborbactam (98.9%), while susceptibility to imipenem-relebactam was lower (93.0%). The cross-combination test using nine combinations of the individual β-lactams (BLs) and new β-lactamase inhibitors (BLIs) showed that the inhibitory activity of avibactam was significantly superior to relebactam or vaborbactam when the Log2 MIC of BLs were compared for each combination with BLIs (all P < 0.05). The dose-escalation test of new BLIs demonstrated that increasing doses of new BLIs corresponded to increased susceptibility to BLs. Taken together, this study illustrates the excellent activity of ceftazidime-avibactam against KPC-2-producing Enterobacterales and suggests further investigation into high-concentration regimens for potentially non-susceptible clinical isolates.
Keywords: KPC; avibactam; relebactam; susceptibility; vaborbactam.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
In vitro activity and genomic characterization of KPC-producing Klebsiella pneumoniae clinical blood culture isolates resistant to ceftazidime/avibactam, meropenem/vaborbactam, imipenem/relebactam: an Italian nationwide multicentre observational study (2022-23).J Antimicrob Chemother. 2025 Feb 3;80(2):583-592. doi: 10.1093/jac/dkae450. J Antimicrob Chemother. 2025. PMID: 39699187
-
Antimicrobial susceptibility of enterobacterales causing bloodstream infection in United States medical centres: comparison of aztreonam-avibactam with beta-lactams active against carbapenem-resistant enterobacterales.BMC Infect Dis. 2024 Nov 5;24(1):1242. doi: 10.1186/s12879-024-10133-5. BMC Infect Dis. 2024. PMID: 39501203 Free PMC article.
-
In vitro activity of ceftazidime-avibactam, ceftolozane-tazobactam, imipenem-relebactam, meropenem-vaborbactam and cefiderocol against carbapenemase-producing Enterobacterales from clinical isolates in a tertiary healthcare centre in Serbia.Acta Microbiol Immunol Hung. 2025 Feb 19;72(1):23-32. doi: 10.1556/030.2025.02521. Print 2025 Mar 27. Acta Microbiol Immunol Hung. 2025. PMID: 39969523
-
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations.Drugs. 2018 Jan;78(1):65-98. doi: 10.1007/s40265-017-0851-9. Drugs. 2018. PMID: 29230684 Review.
-
Non-KPC Attributes of Newer β-lactam/β-lactamase Inhibitors, Part 1: Enterobacterales and Pseudomonas aeruginosa.Clin Infect Dis. 2024 Jul 19;79(1):33-42. doi: 10.1093/cid/ciae048. Clin Infect Dis. 2024. PMID: 38306487 Review.
Cited by
-
Global trends of ceftazidime-avibactam resistance in gram-negative bacteria: systematic review and meta-analysis.Antimicrob Resist Infect Control. 2025 Feb 11;14(1):10. doi: 10.1186/s13756-025-01518-5. Antimicrob Resist Infect Control. 2025. PMID: 39934901 Free PMC article.
References
-
- Lee CM, Lee S, Kim ES, Kim HB, Park WB, Moon SM, Kim YK, Park K-H, Kwak YG, Kim B, Kim YS, Kim C-J, Gil H-Y, Ahn J, Song K-H. 2024. Disease burden of bacteraemia with extended-spectrum beta-lactamase-producing and carbapenem-resistant Enterobacterales in Korea. J Hosp Infect 144:85–93. doi:10.1016/j.jhin.2023.11.013 - DOI - PubMed
-
- Booq RY, Abutarboush MH, Alolayan MA, Huraysi AA, Alotaibi AN, Alturki MI, Alshammari MK, Bakr AA, Alquait AA, Tawfik EA, Alsaleh NB, Bahwerth FS, Alarawi MS, Alyamani EJ, Sendy BK. 2022. Identification and characterization of plasmids and genes from carbapenemase-producing Klebsiella pneumoniae in Makkah Province, Saudi Arabia. Antibiotics (Basel) 11:1627. doi:10.3390/antibiotics11111627 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

