PAR2-mediated cellular senescence promotes inflammation and fibrosis in aging and chronic kidney disease
- PMID: 38687090
- PMCID: PMC11320361
- DOI: 10.1111/acel.14184
PAR2-mediated cellular senescence promotes inflammation and fibrosis in aging and chronic kidney disease
Abstract
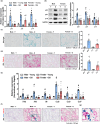
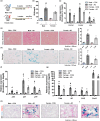
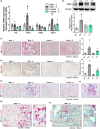
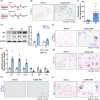
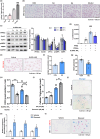
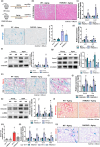
Cellular senescence contributes to inflammatory kidney disease via the secretion of inflammatory and profibrotic factors. Protease-activating receptor 2 (PAR2) is a key regulator of inflammation in kidney diseases. However, the relationship between PAR2 and cellular senescence in kidney disease has not yet been described. In this study, we found that PAR2-mediated metabolic changes in renal tubular epithelial cells induced cellular senescence and increased inflammatory responses. Using an aging and renal injury model, PAR2 expression was shown to be associated with cellular senescence. Under in vitro conditions in NRK52E cells, PAR2 activation induces tubular epithelial cell senescence and senescent cells showed defective fatty acid oxidation (FAO). Cpt1α inhibition showed similar senescent phenotype in the cells, implicating the important role of defective FAO in senescence. Finally, we subjected mice lacking PAR2 to aging and renal injury. PAR2-deficient kidneys are protected from adenine- and cisplatin-induced renal fibrosis and injury, respectively, by reducing senescence and inflammation. Moreover, kidneys lacking PAR2 exhibited reduced numbers of senescent cells and inflammation during aging. These findings offer fresh insights into the mechanisms underlying renal senescence and indicate that targeting PAR2 or FAO may be a promising therapeutic approach for managing kidney injury.
Keywords: PAR2; SASP; aging; fatty acid oxidation; fibrosis; inflammation; senescence.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
-
- Baraibar, M. A. , Hyzewicz, J. , Rogowska‐Wrzesinska, A. , Bulteau, A. L. , Prip‐Buus, C. , Butler‐Browne, G. , & Friguet, B. (2016). Impaired energy metabolism of senescent muscle satellite cells is associated with oxidative modifications of glycolytic enzymes. Aging (Albany NY), 8(12), 3375–3389. 10.18632/aging.101126 - DOI - PMC - PubMed
-
- Chen, P. , Zhang, Q. , Zhang, H. , Gao, Y. , Zhou, Y. , Chen, Y. , Guan, L. , Jiao, T. , Zhao, Y. , Huang, M. , & Bi, H. (2021). Carnitine palmitoyltransferase 1C reverses cellular senescence of MRC‐5 fibroblasts via regulating lipid accumulation and mitochondrial function. Journal of Cellular Physiology, 236(2), 958–970. 10.1002/jcp.29906 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

