Optical Phenomena in Molecule-Based Magnetic Materials
- PMID: 38687182
- PMCID: PMC11082909
- DOI: 10.1021/acs.chemrev.3c00840
Optical Phenomena in Molecule-Based Magnetic Materials
Abstract
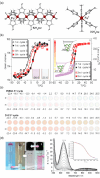
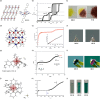
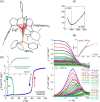
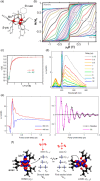
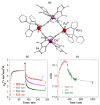
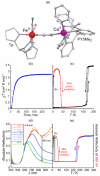
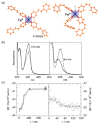
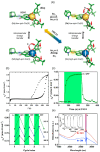
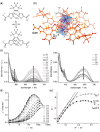
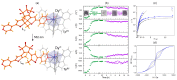
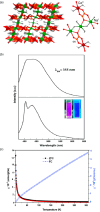
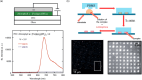
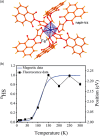
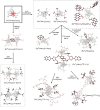
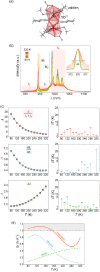
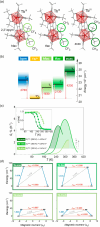
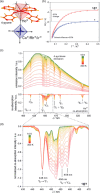
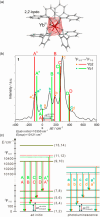
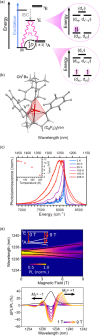
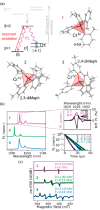
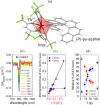
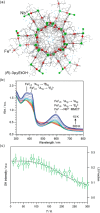
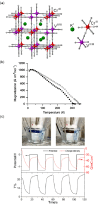
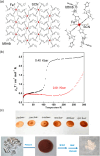
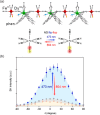
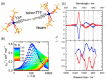
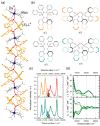
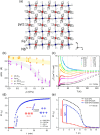
Since the last century, we have witnessed the development of molecular magnetism which deals with magnetic materials based on molecular species, i.e., organic radicals and metal complexes. Among them, the broadest attention was devoted to molecule-based ferro-/ferrimagnets, spin transition materials, including those exploring electron transfer, molecular nanomagnets, such as single-molecule magnets (SMMs), molecular qubits, and stimuli-responsive magnetic materials. Their physical properties open the application horizons in sensors, data storage, spintronics, and quantum computation. It was found that various optical phenomena, such as thermochromism, photoswitching of magnetic and optical characteristics, luminescence, nonlinear optical and chiroptical effects, as well as optical responsivity to external stimuli, can be implemented into molecule-based magnetic materials. Moreover, the fruitful interactions of these optical effects with magnetism in molecule-based materials can provide new physical cross-effects and multifunctionality, enriching the applications in optical, electronic, and magnetic devices. This Review aims to show the scope of optical phenomena generated in molecule-based magnetic materials, including the recent advances in such areas as high-temperature photomagnetism, optical thermometry utilizing SMMs, optical addressability of molecular qubits, magneto-chiral dichroism, and opto-magneto-electric multifunctionality. These findings are discussed in the context of the types of optical phenomena accessible for various classes of molecule-based magnetic materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



























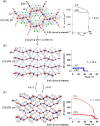



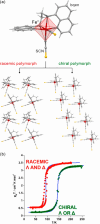
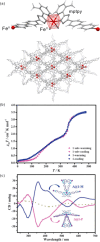




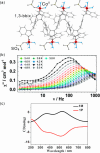




















References
-
- Coronado E. Molecular Magnetism: From Chemical Design to Spin Control in Molecules, Materials and Devices. Nat. Rev. Mater. 2020, 5, 87–104. 10.1038/s41578-019-0146-8. - DOI
-
- Coey J. M. D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131. 10.1016/j.eng.2018.11.034. - DOI
-
- Talaat A.; Suraj M. V.; Byerly K.; Wang A.; Wang Y.; Lee J. K.; Ohodnicki Jr P. R. Review on Soft Magnetic Metal and Inorganic Oxide Nanocomposites for Power Applications. J. Alloys Compd. 2021, 870, 159500.10.1016/j.jallcom.2021.159500. - DOI
Publication types
LinkOut - more resources
Full Text Sources

