Addition of Contrast-enhanced Mammography to Tomosynthesis for Breast Cancer Detection in Women with a Personal History of Breast Cancer: Prospective TOCEM Trial Interim Analysis
- PMID: 38687218
- PMCID: PMC11070607
- DOI: 10.1148/radiol.231991
Addition of Contrast-enhanced Mammography to Tomosynthesis for Breast Cancer Detection in Women with a Personal History of Breast Cancer: Prospective TOCEM Trial Interim Analysis
Abstract
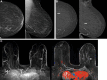
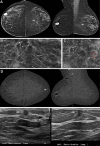
Background Digital breast tomosynthesis (DBT) is often inadequate for screening women with a personal history of breast cancer (PHBC). The ongoing prospective Tomosynthesis or Contrast-Enhanced Mammography, or TOCEM, trial includes three annual screenings with both DBT and contrast-enhanced mammography (CEM). Purpose To perform interim assessment of cancer yield, stage, and recall rate when CEM is added to DBT in women with PHBC. Materials and Methods From October 2019 to December 2022, two radiologists interpreted both examinations: Observer 1 reviewed DBT first and then CEM, and observer 2 reviewed CEM first and then DBT. Effects of adding CEM to DBT on incremental cancer detection rate (ICDR), cancer type and node status, recall rate, and other performance characteristics of the primary radiologist decisions were assessed. Results Among the participants (mean age at entry, 63.6 years ± 9.6 [SD]), 1273, 819, and 227 women with PHBC completed year 1, 2, and 3 screening, respectively. For observer 1, year 1 cancer yield was 20 of 1273 (15.7 per 1000 screenings) for DBT and 29 of 1273 (22.8 per 1000 screenings; ICDR, 7.1 per 1000 screenings [95% CI: 3.2, 13.4]) for DBT plus CEM (P < .001). Year 2 plus 3 cancer yield was four of 1046 (3.8 per 1000 screenings) for DBT and eight of 1046 (7.6 per 1000 screenings; ICDR, 3.8 per 1000 screenings [95% CI: 1.0, 7.6]) for DBT plus CEM (P = .001). Year 1 recall rate for observer 1 was 103 of 1273 (8.1%) for (incidence) DBT alone and 187 of 1273 (14.7%) for DBT plus CEM (difference = 84 of 1273, 6.6% [95% CI: 5.3, 8.1]; P < .001). Year 2 plus 3 recall rate was 40 of 1046 (3.8%) for DBT and 92 of 1046 (8.8%) for DBT plus CEM (difference = 52 of 1046, 5.0% [95% CI: 3.7, 6.3]; P < .001). In 18 breasts with cancer detected only at CEM after integration of both observers, 13 (72%) cancers were invasive (median tumor size, 0.6 cm) and eight of nine (88%) with staging were N0. Among 1883 screenings with adequate reference standard, there were three interval cancers (one at the scar, two in axillae). Conclusion CEM added to DBT increased early breast cancer detection each year in women with PHBC, with an accompanying approximately 5.0%-6.6% recall rate increase. Clinical trial registration no. NCT04085510 © RSNA, 2024 Supplemental material is available for this article.
Conflict of interest statement
Figures


Similar articles
-
Screening for Breast Cancer with Contrast-enhanced Mammography as an Alternative to MRI: SCEMAM Trial Results.Radiology. 2025 Jun;315(3):e242634. doi: 10.1148/radiol.242634. Radiology. 2025. PMID: 40525975 Clinical Trial.
-
Breast Density and Breast Cancer Screening with Digital Breast Tomosynthesis: A TOSYMA Trial Subanalysis.Radiology. 2023 Feb;306(2):e221006. doi: 10.1148/radiol.221006. Epub 2022 Oct 4. Radiology. 2023. PMID: 36194110 Clinical Trial.
-
Digital Mammography, Tomosynthesis, and Contrast-Enhanced Mammography: Intraindividual Comparison of Mean Glandular Dose for Screening Examinations.AJR Am J Roentgenol. 2025 Mar;224(3):e2432150. doi: 10.2214/AJR.24.32150. Epub 2025 Jan 15. AJR Am J Roentgenol. 2025. PMID: 39813603
-
Supplemental Screening as an Adjunct to Mammography for Breast Cancer Screening in People With Dense Breasts: A Health Technology Assessment.Ont Health Technol Assess Ser. 2023 Dec 19;23(9):1-293. eCollection 2023. Ont Health Technol Assess Ser. 2023. PMID: 39364436 Free PMC article.
-
Digital breast tomosynthesis (DBT) plus synthesised two-dimensional mammography (s2D) in breast cancer screening is associated with higher cancer detection and lower recalls compared to digital mammography (DM) alone: results of a systematic review and meta-analysis.Eur Radiol. 2022 Apr;32(4):2301-2312. doi: 10.1007/s00330-021-08308-8. Epub 2021 Oct 25. Eur Radiol. 2022. PMID: 34694451 Free PMC article.
Cited by
-
ESR Essentials: post-treatment breast cancer surveillance from mammography to a more personalised approach-practice recommendations by the European Society of Breast Imaging.Eur Radiol. 2025 Aug 14. doi: 10.1007/s00330-025-11819-3. Online ahead of print. Eur Radiol. 2025. PMID: 40813505 Review.
-
Inter-reader agreement of the BI-RADS CEM lexicon.Eur Radiol. 2025 May;35(5):2378-2386. doi: 10.1007/s00330-024-11176-7. Epub 2024 Nov 6. Eur Radiol. 2025. PMID: 39505735 Free PMC article.
References
-
- Houssami N , Ciatto S , Martinelli F , Bonardi R , Duffy SW . Early detection of second breast cancers improves prognosis in breast cancer survivors . Ann Oncol 2009. ; 20 ( 9 ): 1505 – 1510 . - PubMed
-
- Lowry KP , Braunstein LZ , Economopoulos KP , et al. . Predictors of surveillance mammography outcomes in women with a personal history of breast cancer . Breast Cancer Res Treat 2018. ; 171 ( 1 ): 209 – 215 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

