Diamond-Like Carbon Depositing on the Surface of Polylactide Membrane for Prevention of Adhesion Formation During Tendon Repair
- PMID: 38687411
- PMCID: PMC11061095
- DOI: 10.1007/s40820-024-01392-7
Diamond-Like Carbon Depositing on the Surface of Polylactide Membrane for Prevention of Adhesion Formation During Tendon Repair
Abstract
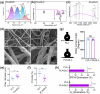
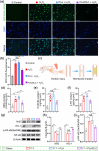
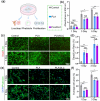
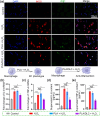
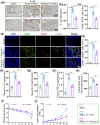
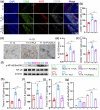
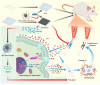
Post-traumatic peritendinous adhesion presents a significant challenge in clinical medicine. This study proposes the use of diamond-like carbon (DLC) deposited on polylactic acid (PLA) membranes as a biophysical mechanism for anti-adhesion barrier to encase ruptured tendons in tendon-injured rats. The results indicate that PLA/DLC composite membrane exhibits more efficient anti-adhesion effect than PLA membrane, with histological score decreasing from 3.12 ± 0.27 to 2.20 ± 0.22 and anti-adhesion effectiveness increasing from 21.61% to 44.72%. Mechanistically, the abundant C=O bond functional groups on the surface of DLC can reduce reactive oxygen species level effectively; thus, the phosphorylation of NF-κB and M1 polarization of macrophages are inhibited. Consequently, excessive inflammatory response augmented by M1 macrophage-originated cytokines including interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) is largely reduced. For biocompatibility evaluation, PLA/DLC membrane is slowly absorbed within tissue and displays prolonged barrier effects compared to traditional PLA membranes. Further studies show the DLC depositing decelerates the release of degradation product lactic acid and its induction of macrophage M2 polarization by interfering esterase and PLA ester bonds, which further delays the fibrosis process. It was found that the PLA/DLC membrane possess an efficient biophysical mechanism for treatment of peritendinous adhesion.
Keywords: Antioxidant; Biodegradation; Diamond-like carbon; Foreign body reaction; Peritendinous adhesion; Reactive oxygen species scavenging.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Yaozhong Zhang is an editorial board member/editor-in-chief for Nano-Micro Letters and was not involved in the editorial review or the decision to publish this article.
Figures


Similar articles
-
Macrophage Polarization Modulated by NF-κB in Polylactide Membranes-Treated Peritendinous Adhesion.Small. 2022 Apr;18(13):e2104112. doi: 10.1002/smll.202104112. Epub 2021 Nov 23. Small. 2022. PMID: 34816589
-
Pyrrolidine Dithiocarbamate-loaded Electrospun Membranes for Peritendinous Anti-adhesion through Inhibition of the Nuclear Factor-κB Pathway.Acta Biomater. 2023 Jan 1;155:333-346. doi: 10.1016/j.actbio.2022.10.004. Epub 2022 Oct 12. Acta Biomater. 2023. PMID: 36243373
-
Macrophage infiltration of electrospun polyester fibers.Biomater Sci. 2017 Jul 25;5(8):1579-1587. doi: 10.1039/c6bm00958a. Biomater Sci. 2017. PMID: 28225101
-
Biomedical applications of diamond-like carbon coatings: a review.J Biomed Mater Res B Appl Biomater. 2007 Oct;83(1):72-84. doi: 10.1002/jbm.b.30768. J Biomed Mater Res B Appl Biomater. 2007. PMID: 17285609 Review.
-
The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis.Front Immunol. 2022 May 19;13:867260. doi: 10.3389/fimmu.2022.867260. eCollection 2022. Front Immunol. 2022. PMID: 35663975 Free PMC article. Review.
Cited by
-
Formation of Nanodiamonds during Pyrolysis of Butanosolv Lignin.ACS Nano. 2024 Sep 10;18(36):24803-24811. doi: 10.1021/acsnano.4c02950. Epub 2024 Aug 23. ACS Nano. 2024. PMID: 39177501 Free PMC article.
-
Healing of tendon-related diseases: insights from macrophage regulation.Mil Med Res. 2025 Aug 4;12(1):45. doi: 10.1186/s40779-025-00635-x. Mil Med Res. 2025. PMID: 40760449 Free PMC article. Review.
-
The roles and mechanisms of the NF-κB signaling pathway in tendon disorders.Front Vet Sci. 2024 Jun 24;11:1382239. doi: 10.3389/fvets.2024.1382239. eCollection 2024. Front Vet Sci. 2024. PMID: 38978635 Free PMC article. Review.
References
-
- M. Lu, S. Wang, H. Wang, T. Xue, C. Cai et al., Pyrrolidine dithiocarbamate-loaded electrospun membranes for peritendinous anti-adhesion through inhibition of the nuclear factor-κB pathway. Acta Biomater. 155, 333–346 (2023). 10.1016/j.actbio.2022.10.004 - PubMed
-
- X. Rong, Y. Tang, S. Cao, S. Xiao, H. Wang et al., An extracellular vesicle-cloaked multifaceted biocatalyst for ultrasound-augmented tendon matrix reconstruction and immune microenvironment regulation. ACS Nano 17, 16501–16516 (2023). 10.1021/acsnano.3c00911 - PubMed
-
- B. Kheilnezhad, A. Hadjizadeh, A review: progress in preventing tissue adhesions from a biomaterial perspective. Biomater. Sci. 9, 2850–2873 (2021). 10.1039/d0bm02023k - PubMed
-
- Y. Li, C. Hu, B. Hu, J. Tian, G. Zhao et al., Sustained release of dicumarol via novel grafted polymer in electrospun nanofiber membrane for treatment of peritendinous adhesion. Adv. Healthc. Mater. 12, e2203078 (2023). 10.1002/adhm.202203078 - PubMed
-
- Y. Dogramaci, A. Kalac, E. Atik, E. Esen, M.E. Altuğ et al., Effects of a single application of extractum cepae on the peritendinous adhesion. Ann. Plast. Surg. 64, 338–341 (2010). 10.1097/sap.0b013e3181afa428 - PubMed
LinkOut - more resources
Full Text Sources
