Dietary dicarboxylic acids provide a nonstorable alternative fat source that protects mice against obesity
- PMID: 38687608
- PMCID: PMC11178532
- DOI: 10.1172/JCI174186
Dietary dicarboxylic acids provide a nonstorable alternative fat source that protects mice against obesity
Abstract
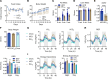
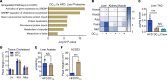
Dicarboxylic fatty acids are generated in the liver and kidney in a minor pathway called fatty acid ω-oxidation. The effects of consuming dicarboxylic fatty acids as an alternative source of dietary fat have not been explored. Here, we fed dodecanedioic acid, a 12-carbon dicarboxylic (DC12), to mice at 20% of daily caloric intake for 9 weeks. DC12 increased metabolic rate, reduced body fat, reduced liver fat, and improved glucose tolerance. We observed DC12-specific breakdown products in liver, kidney, muscle, heart, and brain, indicating that oral DC12 escaped first-pass liver metabolism and was utilized by many tissues. In tissues expressing the "a" isoform of acyl-CoA oxidase-1 (ACOX1), a key peroxisomal fatty acid oxidation enzyme, DC12 was chain shortened to the TCA cycle intermediate succinyl-CoA. In tissues with low peroxisomal fatty acid oxidation capacity, DC12 was oxidized by mitochondria. In vitro, DC12 was catabolized even by adipose tissue and was not stored intracellularly. We conclude that DC12 and other dicarboxylic acids may be useful for combatting obesity and for treating metabolic disorders.
Keywords: Fatty acid oxidation; Metabolism; Mitochondria; Obesity.
Conflict of interest statement
Figures








Comment in
- Dicarboxylic acids counteract the metabolic effects of a Western diet by boosting energy expenditure doi: 10.1172/JCI181978

