Increased hepatic glucose production with lower oxidative metabolism in the growth-restricted fetus
- PMID: 38687612
- PMCID: PMC11141920
- DOI: 10.1172/jci.insight.176497
Increased hepatic glucose production with lower oxidative metabolism in the growth-restricted fetus
Abstract
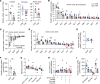
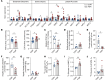
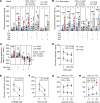
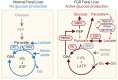
Fetal growth restriction (FGR) is accompanied by early activation of hepatic glucose production (HGP), a hallmark of type 2 diabetes (T2D). Here, we used fetal hepatic catheterization to directly measure HGP and substrate flux in a sheep FGR model. We hypothesized that FGR fetuses would have increased hepatic lactate and amino acid uptake to support increased HGP. Indeed, FGR fetuses compared with normal (CON) fetuses had increased HGP and activation of gluconeogenic genes. Unexpectedly, hepatic pyruvate output was increased, while hepatic lactate and gluconeogenic amino acid uptake rates were decreased in FGR liver. Hepatic oxygen consumption and total substrate uptake rates were lower. In FGR liver tissue, metabolite abundance, 13C-metabolite labeling, enzymatic activity, and gene expression supported decreased pyruvate oxidation and increased lactate production. Isolated hepatocytes from FGR fetuses had greater intrinsic capacity for lactate-fueled glucose production. FGR livers also had lower energy (ATP) and redox state (NADH/NAD+ ratio). Thus, reduced hepatic oxidative metabolism may make carbons available for increased HGP, but also produces nutrient and energetic stress in FGR liver. Intrinsic programming of these pathways regulating HGP in the FGR fetus may underlie increased HGP and T2D risk postnatally.
Keywords: Gluconeogenesis; Reproductive biology.
Conflict of interest statement
Figures



References
-
- Hay WW, Jr Fetal glucose uptake and utilization as functions of maternal glucose concentration. Am J Physiol. 1984;246(3 pt 1):E237–E242. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

