Does Acinetobacter calcoaceticus glucose dehydrogenase produce self-damaging H2O2?
- PMID: 38687614
- PMCID: PMC11130540
- DOI: 10.1042/BSR20240102
Does Acinetobacter calcoaceticus glucose dehydrogenase produce self-damaging H2O2?
Abstract
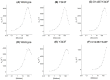
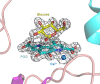
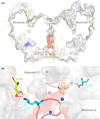
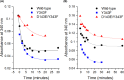
The soluble glucose dehydrogenase (sGDH) from Acinetobacter calcoaceticus has been widely studied and is used, in biosensors, to detect the presence of glucose, taking advantage of its high turnover and insensitivity to molecular oxygen. This approach, however, presents two drawbacks: the enzyme has broad substrate specificity (leading to imprecise blood glucose measurements) and shows instability over time (inferior to other oxidizing glucose enzymes). We report the characterization of two sGDH mutants: the single mutant Y343F and the double mutant D143E/Y343F. The mutants present enzyme selectivity and specificity of 1.2 (Y343F) and 5.7 (D143E/Y343F) times higher for glucose compared with that of the wild-type. Crystallographic experiments, designed to characterize these mutants, surprisingly revealed that the prosthetic group PQQ (pyrroloquinoline quinone), essential for the enzymatic activity, is in a cleaved form for both wild-type and mutant structures. We provide evidence suggesting that the sGDH produces H2O2, the level of production depending on the mutation. In addition, spectroscopic experiments allowed us to follow the self-degradation of the prosthetic group and the disappearance of sGDH's glucose oxidation activity. These studies suggest that the enzyme is sensitive to its self-production of H2O2. We show that the premature aging of sGDH can be slowed down by adding catalase to consume the H2O2 produced, allowing the design of a more stable biosensor over time. Our research opens questions about the mechanism of H2O2 production and the physiological role of this activity by sGDH.
Keywords: hydrogen peroxide production; protein stability; soluble glucose dehydrogenase.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
Ca2+-assisted, direct hydride transfer, and rate-determining tautomerization of C5-reduced PQQ to PQQH2, in the oxidation of beta-D-glucose by soluble, quinoprotein glucose dehydrogenase.Biochemistry. 2000 Aug 8;39(31):9384-92. doi: 10.1021/bi992810x. Biochemistry. 2000. PMID: 10924133
-
Soluble aldose sugar dehydrogenase from Escherichia coli: a highly exposed active site conferring broad substrate specificity.J Biol Chem. 2006 Oct 13;281(41):30650-9. doi: 10.1074/jbc.M601783200. Epub 2006 Jul 24. J Biol Chem. 2006. PMID: 16864586
-
Ca(2+) stabilizes the semiquinone radical of pyrroloquinoline quinone.Biochem J. 2001 Aug 1;357(Pt 3):893-8. doi: 10.1042/0264-6021:3570893. Biochem J. 2001. PMID: 11463363 Free PMC article.
-
Determination of enzyme mechanisms by molecular dynamics: studies on quinoproteins, methanol dehydrogenase, and soluble glucose dehydrogenase.Protein Sci. 2004 Aug;13(8):1965-78. doi: 10.1110/ps.04673404. Protein Sci. 2004. PMID: 15273299 Free PMC article. Review.
-
Physiological significance and bioenergetic aspects of glucose dehydrogenase.Antonie Van Leeuwenhoek. 1989 May;56(1):51-61. doi: 10.1007/BF00822584. Antonie Van Leeuwenhoek. 1989. PMID: 2549864 Review.
References
-
- Wohlfahrt G., Witt S., Hendle J., Schomburg D., Kalisz H.M. and Hecht H.J. (1999) 1.8 and 1.9 Å resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr. D. Biol. Crystallogr. 55, 969–977 10.1107/S0907444999003431 - DOI - PubMed
-
- Yoshida H., Kojima K., Shiota M., Yoshimatsu K., Yamazaki T., Ferri S.et al. . (2019) X-ray structure of the direct electron transfer-type FAD glucose dehydrogenase catalytic subunit complexed with a hitchhiker protein. Acta Crystallogr. Sect. Struct. Biol. 75, 841–851 10.1107/S2059798319010878 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

