Insights into the human metabolism of hexahydrocannabinol by non-targeted liquid chromatography-high-resolution tandem mass spectrometry
- PMID: 38687640
- PMCID: PMC11165647
- DOI: 10.1093/jat/bkae022
Insights into the human metabolism of hexahydrocannabinol by non-targeted liquid chromatography-high-resolution tandem mass spectrometry
Abstract
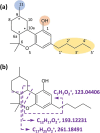
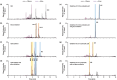
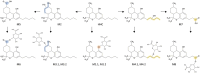
Hexahydrocannabinol (HHC), 6,6,9-trimethyl-3-pentyl-6a,7,8,9,10,10a-hexahydrobenzo[c]chromen-1-ol, is a semi-synthetic cannabinoid that has presented challenges to analytical laboratories due to its emergence and spread in the drug market. The lack of information on human pharmacokinetics hinders the development and application of presumptive and confirmatory tests for reliably detecting HHC consumption. To address this knowledge gap, we report the analytical results obtained from systematic forensic toxicological analysis of body-fluid samples collected from three individuals suspected of drug-impaired driving after HHC consumption. Urine and plasma samples were analyzed using non-targeted liquid chromatography-high-resolution tandem mass spectrometry. The results provided evidence that HHC undergoes biotransformation reactions similar to other well-characterized cannabinoids, such as ∆9-tetrahydrocannabinol or cannabidiol. Notably, HHC itself was only detectable in plasma samples, not in urine samples. The observed Phase I reactions involved oxidation of C11 and the pentyl side chain, leading to corresponding hydroxylated and carboxylic acid species. Additionally, extensive glucuronidation of HHC and its Phase I metabolites was evident.
© The Author(s) 2024. Published by Oxford University Press.
Figures


References
-
- The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) . (2023) Hexahydrocannabinol (HHC) and Related Substances. https://www.emcdda.europa.eu/publications/technical-reports/hhc-and-rela... (accessed May 15, 2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

