The barrier-protective effect of β-eudesmol against type 2-inflammatory cytokine-induced tight junction disassembly in airway epithelial cells
- PMID: 38687777
- PMCID: PMC11060601
- DOI: 10.1371/journal.pone.0302851
The barrier-protective effect of β-eudesmol against type 2-inflammatory cytokine-induced tight junction disassembly in airway epithelial cells
Abstract
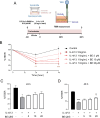
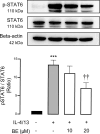
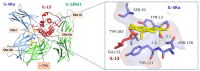
Allergic inflammation, which is the pathogenesis of allergic rhinitis and asthma, is associated with disruption of the airway epithelial barrier due to the effects of type 2 inflammatory cytokines, i.e. interleukin-4 and interleukin-13 (IL-4/13). The anti-allergic inflammatory effect of β-eudesmol (BE) on the tight junction (TJ) of the airway epithelium has not previously been reported. Herein, the barrier protective effect of BE was determined by measurement of transepithelial electrical resistance and by paracellular permeability assay in an IL-4/13-treated 16HBE14o- monolayer. Pre-treatment of BE concentration- and time- dependently inhibited IL-4/13-induced TJ barrier disruption, with the most significant effect observed at 20 μM. Cytotoxicity analyses showed that BE, either alone or in combination with IL-4/13, had no effect on cell viability. Western blot and immunofluorescence analyses showed that BE inhibited IL-4/13-induced mislocalization of TJ components, including occludin and zonula occludens-1 (ZO-1), without affecting the expression of these two proteins. In addition, the mechanism of the TJ-protective effect of BE was mediated by inhibition of IL-4/13-induced STAT6 phosphorylation, in which BE might serve as an antagonist of cytokine receptors. In silico molecular docking analysis demonstrated that BE potentially interacted with the site I pocket of the type 2 IL-4 receptor, likely at Asn-126 and Tyr-127 amino acid residues. It can therefore be concluded that BE is able to prevent IL-4/13-induced TJ disassembly by interfering with cytokine-receptor interaction, leading to suppression of STAT6-induced mislocalization of occludin and ZO-1. BE is a promising candidate for a therapeutic intervention for inflammatory airway epithelial disorders driven by IL-4/13.
Copyright: © 2024 Tharabenjasin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Compound D from Zingiber cassumunar Roxb. attenuated type 2 inflammatory cytokine-induced tight junction disruption in airway epithelial cells.Asian Pac J Allergy Immunol. 2025 Jun;43(2):320-327. doi: 10.12932/AP-180624-1873. Asian Pac J Allergy Immunol. 2025. PMID: 39580628
-
Cholinergic receptor activation on epithelia protects against cytokine-induced barrier dysfunction.Acta Physiol (Oxf). 2015 Apr;213(4):846-59. doi: 10.1111/apha.12469. Acta Physiol (Oxf). 2015. PMID: 25683465
-
Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37.Respir Res. 2019 Nov 9;20(1):251. doi: 10.1186/s12931-019-1226-4. Respir Res. 2019. PMID: 31706310 Free PMC article.
-
IL-1β and the Intestinal Epithelial Tight Junction Barrier.Front Immunol. 2021 Oct 25;12:767456. doi: 10.3389/fimmu.2021.767456. eCollection 2021. Front Immunol. 2021. PMID: 34759934 Free PMC article. Review.
-
Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival.Ann N Y Acad Sci. 2022 Aug;1514(1):21-33. doi: 10.1111/nyas.14798. Epub 2022 May 17. Ann N Y Acad Sci. 2022. PMID: 35580994 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

