Myospreader improves gene editing in skeletal muscle by myonuclear propagation
- PMID: 38687782
- PMCID: PMC11087771
- DOI: 10.1073/pnas.2321438121
Myospreader improves gene editing in skeletal muscle by myonuclear propagation
Abstract
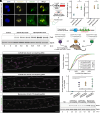
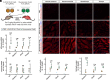
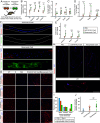
Successful CRISPR/Cas9-based gene editing in skeletal muscle is dependent on efficient propagation of Cas9 to all myonuclei in the myofiber. However, nuclear-targeted gene therapy cargos are strongly restricted to their myonuclear domain of origin. By screening nuclear localization signals and nuclear export signals, we identify "Myospreader," a combination of short peptide sequences that promotes myonuclear propagation. Appending Myospreader to Cas9 enhances protein stability and myonuclear propagation in myoblasts and myofibers. AAV-delivered Myospreader dCas9 better inhibits transcription of toxic RNA in a myotonic dystrophy mouse model. Furthermore, Myospreader Cas9 achieves higher rates of gene editing in CRISPR reporter and Duchenne muscular dystrophy mouse models. Myospreader reveals design principles relevant to all nuclear-targeted gene therapies and highlights the importance of the spatial dimension in therapeutic development.
Keywords: CRISPR; adeno-associated virus; gene therapy; myonuclear domains; spatial gene regulation.
Conflict of interest statement
Competing interests statement:E.T.W. is a co-founder and consultant to Kate Therapeutics. K.K.P. and E.T.W. are inventors on a patent related to this work.
Figures

Update of
-
Myospreader improves gene editing in skeletal muscle by myonuclear propagation.bioRxiv [Preprint]. 2023 Nov 6:2023.11.06.565807. doi: 10.1101/2023.11.06.565807. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 May 7;121(19):e2321438121. doi: 10.1073/pnas.2321438121. PMID: 37986992 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

