Tailored UPRE2 variants for dynamic gene regulation in yeast
- PMID: 38687789
- PMCID: PMC11087760
- DOI: 10.1073/pnas.2315729121
Tailored UPRE2 variants for dynamic gene regulation in yeast
Abstract
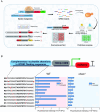
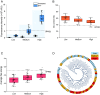
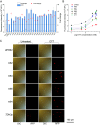
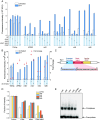
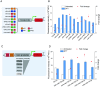
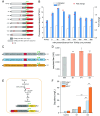
Genetic elements are foundational in synthetic biology serving as vital building blocks. They enable programming host cells for efficient production of valuable chemicals and recombinant proteins. The unfolded protein response (UPR) is a stress pathway in which the transcription factor Hac1 interacts with the upstream unfolded protein response element (UPRE) of the promoter to restore endoplasmic reticulum (ER) homeostasis. Here, we created a UPRE2 mutant (UPRE2m) library. Several rounds of screening identified many elements with enhanced responsiveness and a wider dynamic range. The most active element m84 displayed a response activity 3.72 times higher than the native UPRE2. These potent elements are versatile and compatible with various promoters. Overexpression of HAC1 enhanced stress signal transduction, expanding the signal output range of UPRE2m. Through molecular modeling and site-directed mutagenesis, we pinpointed the DNA-binding residue Lys60 in Hac1(Hac1-K60). We also confirmed that UPRE2m exhibited a higher binding affinity to Hac1. This shed light on the mechanism underlying the Hac1-UPRE2m interaction. Importantly, applying UPRE2m for target gene regulation effectively increased both recombinant protein production and natural product synthesis. These genetic elements provide valuable tools for dynamically regulating gene expression in yeast cell factories.
Keywords: Hac1; UPRE2 variants; responsive promoter; unfolded protein response.
Conflict of interest statement
Competing interests statement:C.X., X.L., Y.P., and M.H. applied a patent for protecting UPRE2m development and its application. The remaining authors declare no competing interest.
Figures
References
-
- Hou J., Tyo K. E., Liu Z., Petranovic D., Nielsen J., Metabolic engineering of recombinant protein secretion by Saccharomyces cerevisiae. FEMS Yeast Res. 12, 491–510 (2012). - PubMed
-
- Idiris A., Tohda H., Kumagai H., Takegawa K., Engineering of protein secretion in yeast: Strategies and impact on protein production. Appl. Microbiol. Biotechnol. 86, 403–417 (2010). - PubMed
-
- Ron D., Walter P., Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

