Brain high-throughput multi-omics data reveal molecular heterogeneity in Alzheimer's disease
- PMID: 38687811
- PMCID: PMC11086901
- DOI: 10.1371/journal.pbio.3002607
Brain high-throughput multi-omics data reveal molecular heterogeneity in Alzheimer's disease
Abstract
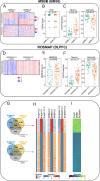
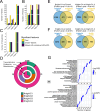
Unbiased data-driven omic approaches are revealing the molecular heterogeneity of Alzheimer disease. Here, we used machine learning approaches to integrate high-throughput transcriptomic, proteomic, metabolomic, and lipidomic profiles with clinical and neuropathological data from multiple human AD cohorts. We discovered 4 unique multimodal molecular profiles, one of them showing signs of poor cognitive function, a faster pace of disease progression, shorter survival with the disease, severe neurodegeneration and astrogliosis, and reduced levels of metabolomic profiles. We found this molecular profile to be present in multiple affected cortical regions associated with higher Braak tau scores and significant dysregulation of synapse-related genes, endocytosis, phagosome, and mTOR signaling pathways altered in AD early and late stages. AD cross-omics data integration with transcriptomic data from an SNCA mouse model revealed an overlapping signature. Furthermore, we leveraged single-nuclei RNA-seq data to identify distinct cell-types that most likely mediate molecular profiles. Lastly, we identified that the multimodal clusters uncovered cerebrospinal fluid biomarkers poised to monitor AD progression and possibly cognition. Our cross-omics analyses provide novel critical molecular insights into AD.
Copyright: © 2024 Eteleeb et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG057777/AG/NIA NIH HHS/United States
- K08 NS101118/NS/NINDS NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- U01 AG072464/AG/NIA NIH HHS/United States
- K01 AG046374/AG/NIA NIH HHS/United States
- R21 NS127211/NS/NINDS NIH HHS/United States
- R01 AG074012/AG/NIA NIH HHS/United States
- R61 NS138655/NS/NINDS NIH HHS/United States
- R01 NS118146/NS/NINDS NIH HHS/United States
- K25 AG083057/AG/NIA NIH HHS/United States
- R56 AG067764/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

