COVID-19 Vaccine Hesitancy: Umbrella Review of Systematic Reviews and Meta-Analysis
- PMID: 38687992
- PMCID: PMC11062401
- DOI: 10.2196/54769
COVID-19 Vaccine Hesitancy: Umbrella Review of Systematic Reviews and Meta-Analysis
Erratum in
-
Corrigendum to: COVID-19 Vaccine Hesitancy: Umbrella Review of Systematic Reviews and Meta-Analysis.JMIR Public Health Surveill. 2024 Jul 10;10:e64080. doi: 10.2196/64080. JMIR Public Health Surveill. 2024. PMID: 38986125 Free PMC article.
Abstract
Background: The unprecedented emergence of the COVID-19 pandemic necessitated the development and global distribution of vaccines, making the understanding of global vaccine acceptance and hesitancy crucial to overcoming barriers to vaccination and achieving widespread immunization.
Objective: This umbrella review synthesizes findings from systematic reviews and meta-analyses to provide insights into global perceptions on COVID-19 vaccine acceptance and hesitancy across diverse populations and regions.
Methods: We conducted a literature search across major databases to identify systematic reviews and meta-analysis that reported COVID-19 vaccine acceptance and hesitancy. The AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews) criteria were used to assess the methodological quality of included systematic reviews. Meta-analysis was performed using STATA 17 with a random effect model. The data synthesis is presented in a table format and via a narrative.
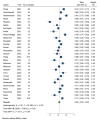
Results: Our inclusion criteria were met by 78 meta-analyses published between 2021 and 2023. Our analysis revealed a moderate vaccine acceptance rate of 63% (95% CI 0.60%-0.67%) in the general population, with significant heterogeneity (I2 = 97.59%). Higher acceptance rates were observed among health care workers and individuals with chronic diseases, at 64% (95% CI 0.57%-0.71%) and 69% (95% CI 0.61%-0.76%), respectively. However, lower acceptance was noted among pregnant women, at 48% (95% CI 0.42%-0.53%), and parents consenting for their children, at 61.29% (95% CI 0.56%-0.67%). The pooled vaccine hesitancy rate was 32% (95% CI 0.25%-0.39%) in the general population. The quality assessment revealed 19 high-quality, 38 moderate-quality, 15 low-quality, and 6 critically low-quality meta-analyses.
Conclusions: This review revealed the presence of vaccine hesitancy globally, emphasizing the necessity for population-specific, culturally sensitive interventions and clear, credible information dissemination to foster vaccine acceptance. The observed disparities accentuate the need for continuous research to understand evolving vaccine perceptions and to address the unique concerns and needs of diverse populations, thereby aiding in the formulation of effective and inclusive vaccination strategies.
Trial registration: PROSPERO CRD42023468363; https://tinyurl.com/2p9kv9cr.
Keywords: COVID-19; child; children; chronic disease; global perceptions; healthcare workers; hesitancy; meta-analysis; parents; patient; patients; perception; pregnant women; random effect model; synthesis; systematic review; umbrella review; vaccine; vaccine acceptance; vaccine hesitancy.
©Tahani Al Rahbeni, Prakasini Satapathy, Ramaiah Itumalla, Roy Rillera Marzo, Khalid A L Mugheed, Mahalaqua Nazli Khatib, Shilpa Gaidhane, Quazi Syed Zahiruddin, Ali A Rabaan, Hayam A Alrasheed, Maha F Al-Subaie, Nawal A Al Kaabil, Mohammed Alissa, Amani Ahmed A L Ibrahim, Hussain Abdulkhaliq Alsaif, Israa Habeeb Naser, Sarvesh Rustagi, Neelima Kukreti, Arkadiusz Dziedzic. Originally published in JMIR Public Health and Surveillance (https://publichealth.jmir.org), 30.04.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Park JJH, Mogg R, Smith GE, Nakimuli-Mpungu E, Jehan F, Rayner CR, Condo J, Decloedt EH, Nachega JB, Reis G, Mills EJ. How COVID-19 has fundamentally changed clinical research in global health. The Lancet Global Health. 2021 May;9(5):e711–e720. doi: 10.1016/s2214-109x(20)30542-8. - DOI - PMC - PubMed
-
- Omer SB, Benjamin RM, Brewer NT, Buttenheim AM, Callaghan T, Caplan A, Carpiano RM, Clinton C, DiResta R, Elharake JA, Flowers LC, Galvani AP, Lakshmanan R, Maldonado YA, McFadden SM, Mello MM, Opel DJ, Reiss DR, Salmon DA, Schwartz JL, Sharfstein JM, Hotez PJ. Promoting COVID-19 vaccine acceptance: recommendations from the Lancet Commission on Vaccine Refusal, Acceptance, and Demand in the USA. The Lancet. 2021 Dec;398(10317):2186–2192. doi: 10.1016/s0140-6736(21)02507-1. - DOI - PMC - PubMed
-
- National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division. Board on Health Sciences Policy. Board on Population Health and Public Health Practice. Committee on Equitable Allocation of Vaccine for the Novel Coronavirus. Gayle H, Foege W, Brown L, Kahn B. Framework for equitable allocation of COVID-19 vaccine. National Academies Press. 2020. [2024-03-09]. https://nap.nationalacademies.org/catalog/25917/framework-for-equitable-... . - PubMed
-
- Gozzi N, Chinazzi M, Dean NE, Longini IM, Halloran ME, Perra N, Vespignani A. Estimating the impact of COVID-19 vaccine inequities: a modeling study. Nat Commun. 2023 Jun 06;14(1):3272. doi: 10.1038/s41467-023-39098-w. doi: 10.1038/s41467-023-39098-w.10.1038/s41467-023-39098-w - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

