Selective refueling of CAR T cells using ADA1 and CD26 boosts antitumor immunity
- PMID: 38688275
- PMCID: PMC11148642
- DOI: 10.1016/j.xcrm.2024.101530
Selective refueling of CAR T cells using ADA1 and CD26 boosts antitumor immunity
Abstract
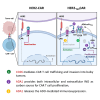
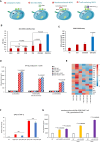
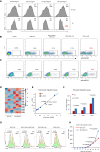
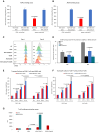
Chimeric antigen receptor (CAR) T cell therapy is hindered in solid tumor treatment due to the immunosuppressive tumor microenvironment and suboptimal T cell persistence. Current strategies do not address nutrient competition in the microenvironment. Hence, we present a metabolic refueling approach using inosine as an alternative fuel. CAR T cells were engineered to express membrane-bound CD26 and cytoplasmic adenosine deaminase 1 (ADA1), converting adenosine to inosine. Autocrine secretion of ADA1 upon CD3/CD26 stimulation activates CAR T cells, improving migration and resistance to transforming growth factor β1 suppression. Fusion of ADA1 with anti-CD3 scFv further boosts inosine production and minimizes tumor cell feeding. In mouse models of hepatocellular carcinoma and non-small cell lung cancer, metabolically refueled CAR T cells exhibit superior tumor reduction compared to unmodified CAR T cells. Overall, our study highlights the potential of selective inosine refueling to enhance CAR T therapy efficacy against solid tumors.
Keywords: ADA; ADA1 autocrine secretion; CAR T cell; CD26; T cell engager; adenosine; anti-CD3 scFv; inosine; metabolic reprogramming; solid tumor.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests X.S., A.S., and Y.H. are the inventors of the technology discussed in this work, and Texas A&M University has ownership of the technology and has filed a patent application for it. X.S., K.S., and A.S. have equity interests in Cellula Biopharma, Inc., the company that intends to license and commercialize the technology discussed in this work from Texas A&M University.
Figures


Similar articles
-
Protocol for preparing metabolically reprogrammed human CAR T cells and evaluating their in vitro effects.STAR Protoc. 2024 Dec 20;5(4):103333. doi: 10.1016/j.xpro.2024.103333. Epub 2024 Sep 21. STAR Protoc. 2024. PMID: 39306853 Free PMC article.
-
Adenosine Deaminase 1 Overexpression Enhances the Antitumor Efficacy of Chimeric Antigen Receptor-Engineered T Cells.Hum Gene Ther. 2022 Mar;33(5-6):223-236. doi: 10.1089/hum.2021.050. Epub 2021 Aug 25. Hum Gene Ther. 2022. PMID: 34225478 Free PMC article.
-
Developing CAR-T/NK cells that target EphA2 for non-small cell lung cancer treatment.Front Immunol. 2025 Mar 13;16:1448438. doi: 10.3389/fimmu.2025.1448438. eCollection 2025. Front Immunol. 2025. PMID: 40181964 Free PMC article.
-
Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives.Cancer Immunol Immunother. 2021 Mar;70(3):619-631. doi: 10.1007/s00262-020-02735-0. Epub 2020 Oct 6. Cancer Immunol Immunother. 2021. PMID: 33025047 Free PMC article. Review.
-
Chimeric antigen receptor-engineered T-cell therapy for liver cancer.Hepatobiliary Pancreat Dis Int. 2018 Aug;17(4):301-309. doi: 10.1016/j.hbpd.2018.05.005. Epub 2018 May 24. Hepatobiliary Pancreat Dis Int. 2018. PMID: 29861325 Review.
Cited by
-
Advances in research on factors affecting chimeric antigen receptor T-cell efficacy.Cancer Med. 2024 Jun;13(11):e7375. doi: 10.1002/cam4.7375. Cancer Med. 2024. PMID: 38864474 Free PMC article. Review.
-
Bioengineering the metabolic network of CAR T cells with GLP-1 and Urolithin A increases persistence and long-term anti-tumor activity.Cell Rep Med. 2025 Mar 18;6(3):102021. doi: 10.1016/j.xcrm.2025.102021. Cell Rep Med. 2025. PMID: 40107240 Free PMC article.
-
Liquid biopsy using plasma proteomics in predicting efficacy and tolerance of PD-1/PD-L1 blockades in NSCLC: a prospective exploratory study.Mol Biomed. 2025 Jul 15;6(1):51. doi: 10.1186/s43556-025-00291-6. Mol Biomed. 2025. PMID: 40659985 Free PMC article.
-
Hypoxic link between cancer cells and the immune system: The role of adenosine and lactate.Oncol Res. 2025 Jul 18;33(8):1803-1818. doi: 10.32604/or.2025.065953. eCollection 2025. Oncol Res. 2025. PMID: 40746883 Free PMC article. Review.
-
Applying metabolic control strategies to engineered T cell cancer therapies.Metab Eng. 2024 Nov;86:250-261. doi: 10.1016/j.ymben.2024.10.009. Epub 2024 Oct 25. Metab Eng. 2024. PMID: 39490640 Review.
References
-
- Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M., et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008226. - DOI - PMC - PubMed
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. - DOI - PMC - PubMed
-
- Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X., et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf8621. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

