Time-domain methods for quantifying dynamic cerebral blood flow autoregulation: Review and recommendations. A white paper from the Cerebrovascular Research Network (CARNet)
- PMID: 38688529
- PMCID: PMC11418733
- DOI: 10.1177/0271678X241249276
Time-domain methods for quantifying dynamic cerebral blood flow autoregulation: Review and recommendations. A white paper from the Cerebrovascular Research Network (CARNet)
Abstract
Cerebral Autoregulation (CA) is an important physiological mechanism stabilizing cerebral blood flow (CBF) in response to changes in cerebral perfusion pressure (CPP). By maintaining an adequate, relatively constant supply of blood flow, CA plays a critical role in brain function. Quantifying CA under different physiological and pathological states is crucial for understanding its implications. This knowledge may serve as a foundation for informed clinical decision-making, particularly in cases where CA may become impaired. The quantification of CA functionality typically involves constructing models that capture the relationship between CPP (or arterial blood pressure) and experimental measures of CBF. Besides describing normal CA function, these models provide a means to detect possible deviations from the latter. In this context, a recent white paper from the Cerebrovascular Research Network focused on Transfer Function Analysis (TFA), which obtains frequency domain estimates of dynamic CA. In the present paper, we consider the use of time-domain techniques as an alternative approach. Due to their increased flexibility, time-domain methods enable the mitigation of measurement/physiological noise and the incorporation of nonlinearities and time variations in CA dynamics. Here, we provide practical recommendations and guidelines to support researchers and clinicians in effectively utilizing these techniques to study CA.
Keywords: CARNet; cerebral autoregulation; cerebral blood flow; time-domain methods; white paper.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

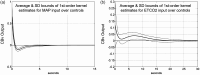
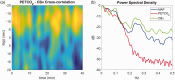
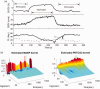
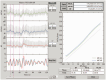
Similar articles
-
Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network.J Cereb Blood Flow Metab. 2016 Apr;36(4):665-80. doi: 10.1177/0271678X15626425. Epub 2016 Jan 18. J Cereb Blood Flow Metab. 2016. PMID: 26782760 Free PMC article. Review.
-
Monitoring of cerebral blood flow autoregulation: physiologic basis, measurement, and clinical implications.Br J Anaesth. 2024 Jun;132(6):1260-1273. doi: 10.1016/j.bja.2024.01.043. Epub 2024 Mar 12. Br J Anaesth. 2024. PMID: 38471987 Review.
-
Noninvasive optical measurement of microvascular cerebral hemodynamics and autoregulation in the neonatal ECMO patient.Pediatr Res. 2020 Dec;88(6):925-933. doi: 10.1038/s41390-020-0841-6. Epub 2020 Mar 14. Pediatr Res. 2020. PMID: 32172282 Free PMC article.
-
Reliability, asymmetry, and age influence on dynamic cerebral autoregulation measured by spontaneous fluctuations of blood pressure and cerebral blood flow velocities in healthy individuals.J Neuroimaging. 2014 Jul-Aug;24(4):379-86. doi: 10.1111/jon.12019. Epub 2013 Apr 22. J Neuroimaging. 2014. PMID: 23607680 Free PMC article.
-
The Effect of Data Length on the Assessment of Dynamic Cerebral Autoregulation with Transfer Function Analysis in Neurological ICU Patients.Neurocrit Care. 2022 Feb;36(1):21-29. doi: 10.1007/s12028-021-01301-5. Epub 2021 Aug 17. Neurocrit Care. 2022. PMID: 34403122 Free PMC article.
Cited by
-
Examining the upper frequency limit of dynamic cerebral autoregulation: Considerations across the cardiac cycle during eucapnia.Exp Physiol. 2024 Dec;109(12):2100-2121. doi: 10.1113/EP091719. Epub 2024 Oct 9. Exp Physiol. 2024. PMID: 39382938 Free PMC article.
-
The pulsing brain: state of the art and an interdisciplinary perspective.Interface Focus. 2025 Apr 4;15(1):20240058. doi: 10.1098/rsfs.2024.0058. eCollection 2025 Apr 4. Interface Focus. 2025. PMID: 40191028 Free PMC article. Review.
-
Brain Ultrasonography in Critically Ill Septic Patients: A Scoping Review.J Clin Med. 2024 Nov 17;13(22):6920. doi: 10.3390/jcm13226920. J Clin Med. 2024. PMID: 39598064 Free PMC article.
-
Individualized mean arterial pressure targets in critically ill patients guided by non-invasive cerebral-autoregulation: a scoping review.Crit Care. 2025 May 16;29(1):196. doi: 10.1186/s13054-025-05432-5. Crit Care. 2025. PMID: 40380314 Free PMC article.
-
The effect of hypercapnia on the directional sensitivity of dynamic cerebral autoregulation and the influence of age and sex.J Cereb Blood Flow Metab. 2024 Feb;44(2):272-283. doi: 10.1177/0271678X231203475. Epub 2023 Sep 25. J Cereb Blood Flow Metab. 2024. PMID: 37747437 Free PMC article.
References
-
- Lewis PM, Smielewski P, Pickard JD, et al.. Dynamic cerebral autoregulation: should intracranial pressure be taken into account? Acta Neurochir (Wien) 2007; 149: 549–555. - PubMed
-
- Czosnyka M, Smielewski P, Kirkpatrick P, et al.. Monitoring of cerebral autoregulation in head-injured patients. Stroke 1996; 27: 1829–1834. - PubMed
-
- Ma H, Guo Z-N, Liu J, et al.. Temporal course of dynamic cerebral autoregulation in patients with intracerebral hemorrhage. Stroke 2016; 47: 674–681. - PubMed
-
- McConnell FK, Payne S. The dual role of cerebral autoregulation and collateral flow in the circle of willis after major vessel occlusion. IEEE Trans Biomed Eng 2016; 64: 1793–1802. - PubMed
-
- Spengos K, Tsivgoulis G, Zakopoulos N. Blood pressure management in acute stroke: a long-standing debate. Eur Neurol 2006; 55: 123–135. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

