High-Risk Extubation Readiness Testing for Children With Cardiac Critical Illness
- PMID: 38688549
- PMCID: PMC11349595
- DOI: 10.4187/respcare.11670
High-Risk Extubation Readiness Testing for Children With Cardiac Critical Illness
Abstract
Background: A protocolized extubation readiness test (ERT), including a spontaneous breathing trial (SBT), is recommended for patients who are intubated. This quality-improvement project aimed to improve peri-extubation outcomes by using a high-risk ERT protocol in intubated cardiac patients in addition to a standard-risk protocol.
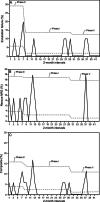
Methods: After baseline data collection, we implemented a standard-risk ERT protocol (pressure support plus PEEP), followed by a high-risk ERT protocol (PEEP alone) in cardiac subjects who were intubated. The primary outcome, a composite of extubation failure and rescue noninvasive respiratory support, was compared between phases. Ventilator duration and use of postextubation respiratory support were balancing measures.
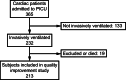
Results: A total of 213 cardiac subjects who were intubated were studied, with extubation failure and rescue noninvasive respiratory support occurring in 10 of 213 (4.7%) and 8 of 213 (3.8%), respectively. We observed a reduction in the composite outcome among the 3 consecutive phases (5/29 [17.2%], 10/110 [9.1%] vs 3/74 [4.1%]; P = .10), but this did not reach statistical significance. In the logistic regression model when adjusting for admission type, the high-risk ERT protocol was associated with a significant reduction of the composite outcome (adjusted odds ratio 0.20, 95% CI 0.04-0.091; P = .037), whereas the standard-risk ERT protocol was not (adjusted odds ratio 0.48, 95% CI 0.15-1.53; P = .21). This was not accompanied by a longer ventilator duration (2.0 [1.0, 3.0], 2.0 [1.0-4.0], vs adjusted odds ratio 2.0 [95% [1.0-6.0]; P = .99) or an increased use of planned noninvasive respiratory support (10/29 [35.5%], 35/110 [31.8%], vs 25/74 [33.8%]; P > .99).
Conclusions: In this quality-improvement project, a high-risk ERT protocol was implemented with improvement in peri-extubation outcomes among cardiac subjects.
Keywords: airway extubation; congenital heart defects; extubation failure; extubation readiness test; mechanical ventilation; respiratory therapy; spontaneous breathing trial; ventilator weaning; weaning failure.
Copyright © 2024 by Daedalus Enterprises.
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Figures
Similar articles
-
Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation.Cochrane Database Syst Rev. 2017 Feb 1;2(2):CD003212. doi: 10.1002/14651858.CD003212.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jul 27;7:CD003212. doi: 10.1002/14651858.CD003212.pub4. PMID: 28146296 Free PMC article. Updated.
-
Noninvasive positive-pressure ventilation as a weaning strategy for intubated adults with respiratory failure.Cochrane Database Syst Rev. 2013 Dec 9;2013(12):CD004127. doi: 10.1002/14651858.CD004127.pub3. Cochrane Database Syst Rev. 2013. PMID: 24323843 Free PMC article.
-
Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation.Cochrane Database Syst Rev. 2014 Sep 4;(9):CD003212. doi: 10.1002/14651858.CD003212.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Feb 01;2:CD003212. doi: 10.1002/14651858.CD003212.pub3. PMID: 25188554 Updated.
-
Cough augmentation techniques for extubation or weaning critically ill patients from mechanical ventilation.Cochrane Database Syst Rev. 2017 Jan 11;1(1):CD011833. doi: 10.1002/14651858.CD011833.pub2. Cochrane Database Syst Rev. 2017. PMID: 28075489 Free PMC article.
-
Effect of Reintubation Within 48 Hours on Mortality in Critically Ill Patients After Planned Extubation.Respir Care. 2024 Jun 28;69(7):829-838. doi: 10.4187/respcare.11077. Respir Care. 2024. PMID: 38772683 Free PMC article. Clinical Trial.
References
-
- Abu-Sultaneh S, Iyer NP, Fernández A, Gaies M, González-Dambrauskas S, Hotz JC, et al. Executive summary: international clinical practice guidelines for pediatric ventilator liberation, a Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network document. Am J Respir Crit Care Med 2023;207(1):17-28. - PMC - PubMed
-
- Foronda FK, Troster EJ, Farias JA, Barbas CS, Ferraro AA, Faria LS, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Crit Care Med 2011;39(11):2526-2533. - PubMed
-
- Tan HL, Ma Y-J, Aguilan AB, Goh CY, Wong JCK, Ang LSL, et al. Respiratory therapist-driven extubation readiness testing in a single pediatric ICU. Respir Care 2022;67(7):833-841. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

