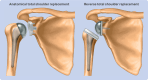
Reverse total shoulder replacement versus anatomical total shoulder replacement for osteoarthritis: population based cohort study using data from the National Joint Registry and Hospital Episode Statistics for England
- PMID: 38688550
- PMCID: PMC11058468
- DOI: 10.1136/bmj-2023-077939
Reverse total shoulder replacement versus anatomical total shoulder replacement for osteoarthritis: population based cohort study using data from the National Joint Registry and Hospital Episode Statistics for England
Abstract
Objectives: To answer a national research priority by comparing the risk-benefit and costs associated with reverse total shoulder replacement (RTSR) and anatomical total shoulder replacement (TSR) in patients having elective primary shoulder replacement for osteoarthritis.
Design: Population based cohort study using data from the National Joint Registry and Hospital Episode Statistics for England.
Setting: Public hospitals and publicly funded procedures at private hospitals in England, 2012-20.
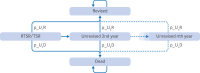
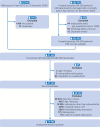
Participants: Adults aged 60 years or older who underwent RTSR or TSR for osteoarthritis with intact rotator cuff tendons. Patients were identified from the National Joint Registry and linked to NHS Hospital Episode Statistics and civil registration mortality data. Propensity score matching and inverse probability of treatment weighting were used to balance the study groups.
Main outcome measures: The main outcome measure was revision surgery. Secondary outcome measures included serious adverse events within 90 days, reoperations within 12 months, prolonged hospital stay (more than three nights), change in Oxford Shoulder Score (preoperative to six month postoperative), and lifetime costs to the healthcare service.
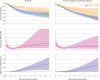
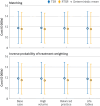
Results: The propensity score matched population comprised 7124 RTSR or TSR procedures (126 were revised), and the inverse probability of treatment weighted population comprised 12 968 procedures (294 were revised) with a maximum follow-up of 8.75 years. RTSR had a reduced hazard ratio of revision in the first three years (hazard ratio local minimum 0.33, 95% confidence interval 0.18 to 0.59) with no clinically important difference in revision-free restricted mean survival time, and a reduced relative risk of reoperations at 12 months (odds ratio 0.45, 95% confidence interval 0.25 to 0.83) with an absolute risk difference of -0.51% (95% confidence interval -0.89 to -0.13). Serious adverse events and prolonged hospital stay risks, change in Oxford Shoulder Score, and modelled mean lifetime costs were similar. Outcomes remained consistent after weighting.
Conclusions: This study's findings provide reassurance that RTSR is an acceptable alternative to TSR for patients aged 60 years or older with osteoarthritis and intact rotator cuff tendons. Despite a significant difference in the risk profiles of revision surgery over time, no statistically significant and clinically important differences between RTSR and TSR were found in terms of long term revision surgery, serious adverse events, reoperations, prolonged hospital stay, or lifetime healthcare costs.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institute for Health and Care Research (NIHR) and the NIHR Oxford Biomedical Research Centre (BRC) for the submitted work. EMV is a NIHR doctoral fellowship award holder. RPV is coapplicant on research grants to the NIHR to the University of Oxford. MRW is principal investigator or coapplicant on research grants to NIHR to the University of Bristol. JLR holds an NIHR senior investigator award and is immediate past president of the British Elbow and Shoulder Society. GSC is an NIHR senior investigator. MRW, AS, and JLR hold a contract with the National Joint Registry (FTS 010307-2022: Statistical Analysis, Support and Associated Services). GC is patient representative for the National Joint Registry. DPA receives funding from the NIHR in the form of a senior research fellowship and from the Oxford NIHR Biomedical Research Centre. DPA’s research group has received research grants from the European Medicines Agency, the Innovative Medicines Initiative, Amgen, Chiesi, and UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, AstraZeneca, and UCB Biopharma. All other authors declare no conflicts of interest.
Figures

Comment in
-
Choosing the best shoulder replacement.BMJ. 2024 Apr 30;385:q952. doi: 10.1136/bmj.q952. BMJ. 2024. PMID: 38688527 No abstract available.
References
-
- National Joint Registry Editorial Committee. 20th Annual Report 2023. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2020th%20Ann... - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
