Impact of Neoadjuvant Radiochemotherapy on Pathological Complete Response for Locally Advanced Rectal Cancer: A Mono Institutional Experience
- PMID: 38688610
- PMCID: PMC11059880
- DOI: 10.21873/invivo.13577
Impact of Neoadjuvant Radiochemotherapy on Pathological Complete Response for Locally Advanced Rectal Cancer: A Mono Institutional Experience
Abstract
Background/aim: Neoadjuvant radiochemotherapy followed by surgery is a standard of care in locally advanced rectal cancer (LARC). Only a subgroup of patients can obtain a pathological complete response (pCR) and achieve good local control. However, the role of pCR on patient survival is debated. The aim of the study was to evaluate the impact of pCR on clinical outcomes and toxicities in LARC patients treated with dose intensification and concomitant capecitabine treatment in a neoadjuvant radiochemotherapy schedule.
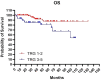
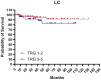
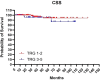
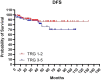
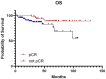
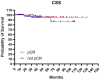
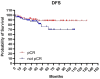
Patients and methods: This was a single Institution retrospective study including 178 patients. Mandard tumor regression grade (TRG) and pTNM staging system were used to classify pathological response and define pathological complete response (pCR). Patients were divided in: pCR (pT0N0) and Not-pCR (pT>0N>0), according to pTNM and in good responders (TRG1-2) and partial/not responders (TRG3-5), according to Mandard TRG. The Kaplan-Meier method was used to estimate OS, CSS, DFS and LC.
Results: A low severe toxicity rate was observed. Acute Grade 3 lower bowel toxicity and Grade 3 cutaneous toxicity were reported in 2 (1.1%) patients, respectively. Late Grade >3 lower bowel toxicity was reported in 6 patients (3%) and late Grade >3 cutaneous toxicity was registered in one patient. No other severe acute and late toxicities were reported. The 5- and 10-year OS, CSS, DFS and LC rates were 85% and 75%, 94% and 92%, 83% and 81%, 88% and 88%, respectively. We observed a pCR rate of 36% and a good responders rate of 62%, in our study population. Both groups showed better rates for each analyzed clinical outcome.
Conclusion: Neoadjuvant radiochemotherapy with dose intensification in LARC patients resulted in favorable long-term oncological outcomes, pCR rate showed an optimal impact on OS and DFS with an acceptable toxicity.
Keywords: Pathological complete response; long-term outcomes; rectal cancer; toxicity.
Copyright © 2024, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare no conflicts of interest associated with the study.
Figures
References
-
- European Cancer Information System: Colonrectal cancer burden in EU-27. Available at: https://ecis.jrc.ec.europa.eu/pdf/Colorectal_cancer_factsheet-Mar_2021.pdf. [Last accessed on January 15, 2021]
-
- Associazione Italiana Registro Tumori, Associazione italiana di Oncologia Medica: i numeri del cancro 2020. Available at: https://www.registri-tumori.it/cms/sites/default/files/pubblicazioni/202.... [Last accessed on October, 2020]
-
- Associazione Italiana Registro Tumori, Associazione italiana di Oncologia Medica: i numeri del cancro 2019. Available at: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operat.... [Last accessed on September, 2020]
-
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
