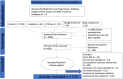
Analysis of core outcome set reporting in coronary intervention trials
- PMID: 38688715
- PMCID: PMC11086530
- DOI: 10.1136/openhrt-2023-002581
Analysis of core outcome set reporting in coronary intervention trials
Abstract
Background: This paper will focus on outcome reporting within percutaneous coronary intervention (PCI) trials. A core outcome set (COS) is a standardised set of outcomes that are recommended to be reported in every clinical trial. Using a COS can help to ensure that all relevant outcomes are consistently reported across clinical trials. In 2018, the European Society of Cardiology outlined the only COS published for PCI trials.
Methods: We searched the literature for all randomised controlled trials published between 2014 and 2022. PCI trials included were late-phase trials and must investigate coronary intervention. The primary outcome was the proportion of trials that reported all of the COS-defined outcomes within their publication as either a primary, secondary or safety endpoint. The secondary outcomes included; the number of primary outcomes reported per study, the proportion of studies which use patient and public involvement (PPI) during trial design, outcome variability and outcome consistency.
Results: 9580 trials were screened and 115 studies met inclusion/exclusion criteria. Our study demonstrated that 55% (34/62) of PCI trials used a COS when it was available, compared with 40% (21/53) before the availability of a PCI COS set, p=0.121. Fewer primary outcomes were reported after the implementation of the COS, 2 compared with 2.3, p=0.014. There was no difference in the use of PPI between either group. There was a higher level of variability in outcomes reported before the availability of the COS, while the consistency of outcome reporting remained similar.
Conclusion: The use of a COS in PCI trials is low. This study provides evidence that there still is a lack of awareness of the COS among those who design clinical trials. We also presented the inconsistency and heterogenicity in reporting clinical trial outcomes. Finally, there was a clear lack of PPI utilisation in PCI trials.
Keywords: Acute Coronary Syndrome; Biostatistics; Coronary Vessels; Myocardial Infarction; Outcome Assessment, Health Care.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Assessing the uptake of the core outcome set in randomized controlled trials for coronary artery disease: a trial registry analysis.Trials. 2025 Feb 25;26(1):66. doi: 10.1186/s13063-025-08765-2. Trials. 2025. PMID: 39994760 Free PMC article. Review.
-
Critical Appraisal of Contemporary Clinical Endpoint Definitions in Coronary Intervention Trials: A Guidance Document.JACC Cardiovasc Interv. 2019 May 13;12(9):805-819. doi: 10.1016/j.jcin.2018.12.031. JACC Cardiovasc Interv. 2019. PMID: 31072504 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Development of a core outcome set for myocardial infarction in clinical trials of traditional Chinese medicine: a study protocol.BMJ Open. 2019 Dec 3;9(12):e032256. doi: 10.1136/bmjopen-2019-032256. BMJ Open. 2019. PMID: 31796484 Free PMC article.
-
Selecting Core Outcomes for Randomised Effectiveness trials In Type 2 Diabetes (SCORE-IT): study protocol for the development of a core outcome set.Trials. 2018 Aug 7;19(1):427. doi: 10.1186/s13063-018-2805-2. Trials. 2018. PMID: 30086771 Free PMC article.
References
-
- Mansoor A, Mehta P, Kumar A, et al. Percutaneous coronary intervention. 2022.
-
- Ferreira-González I, Busse JW, Heels-Ansdell D, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ. 2007;334:786. doi: 10.1136/bmj.39136.682083.AE. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous