Comparative Anatomy of the Dentate Mossy Cells in Nonhuman Primates: Their Spatial Distributions and Axonal Projections Compared With Mouse Mossy Cells
- PMID: 38688719
- PMCID: PMC11151194
- DOI: 10.1523/ENEURO.0151-24.2024
Comparative Anatomy of the Dentate Mossy Cells in Nonhuman Primates: Their Spatial Distributions and Axonal Projections Compared With Mouse Mossy Cells
Abstract
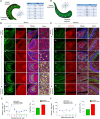
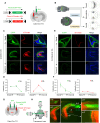
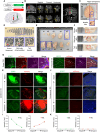
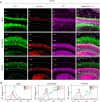
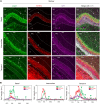
Glutamatergic mossy cells (MCs) mediate associational and commissural connectivity, exhibiting significant heterogeneity along the septotemporal axis of the mouse dentate gyrus (DG). However, it remains unclear whether the neuronal features of MCs are conserved across mammals. This study compares the neuroanatomy of MCs in the DG of mice and monkeys. The MC marker, calretinin, distinguishes two subpopulations: septal and temporal. Dual-colored fluorescence labeling is utilized to compare the axonal projection patterns of these subpopulations. In both mice and monkeys, septal and temporal MCs project axons across the longitudinal axis of the ipsilateral DG, indicating conserved associational projections. However, unlike in mice, no MC subpopulations in monkeys make commissural projections to the contralateral DG. In monkeys, temporal MCs send associational fibers exclusively to the inner molecular layer, while septal MCs give rise to wide axonal projections spanning multiple molecular layers, akin to equivalent MC subpopulations in mice. Despite conserved septotemporal heterogeneity, interspecies differences are observed in the topological organization of septal MCs, particularly in the relative axonal density in each molecular layer along the septotemporal axis of the DG. In summary, this comparative analysis sheds light on both conserved and divergent features of MCs in the DG of mice and monkeys. These findings have implications for understanding functional differentiation along the septotemporal axis of the DG and contribute to our knowledge of the anatomical evolution of the DG circuit in mammals.
Keywords: associational projections; commissural projections; dentate gyrus; heterogeneity; hippocampus; mossy cells.
Copyright © 2024 Jeong et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
