Albuminuria predicts kidney events in IgA nephropathy
- PMID: 38688876
- PMCID: PMC11997806
- DOI: 10.1093/ndt/gfae085
Albuminuria predicts kidney events in IgA nephropathy
Abstract
Background and hypothesis: KDIGO recommends proteinuria <1 g/d as a treatment target in patients with immunoglobulin A nephropathy (IgAN) because of high risk of progression to kidney failure. However, long-term kidney outcomes in patients with low-grade proteinuria remain insufficiently studied.
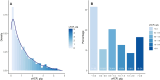
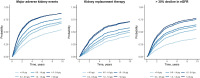
Methods: We enrolled patients with biopsy-proven primary IgAN from the Swedish Renal Registry and analyzed associations between urine albumin-to-creatinine ratio (uACR, in categories <0.3, 0.3-0.5, 0.5-1.0, 1.0-1.5, 1.5-2.0, and ≥2.0 g/g) and the occurrence of major adverse kidney events [MAKE, a composite of kidney replacement therapy (KRT) and >30% decline in estimated glomerular filtration rate (eGFR)]. We also explored the risk of kidney events associated with change in uACR within a year.
Results: We included 1269 IgAN patients (74% men, median 53 years, mean eGFR 33 ml/min/1.73 m², median uACR 0.7 g/g). Over a median follow-up of 5.5 [2.8; 9.2] years, 667 MAKE and 517 KRT events occurred, and 528 patients experienced >30% eGFR decline. Compared with uACR < 0.3 g/g, any higher uACR category was strongly and incrementally associated with the risk of MAKE [adjusted hazard ratios (HR) ranging from 1.56 (95%CI 1.14-2.14) if uACR 0.3-0.5 g/g to 4.53 (3.36-6.11) if uACR ≥ 2.0 g/g], KRT (HR ranging from 1.39 to 4.65), and eGFR decline >30% (HR ranging from 1.76 to 3.47). In 785 patients who had repeated uACR measurements within a year, and compared with stable uACR, the risk of kidney events was lower if uACR decreased by 2-fold (HR ranging from 0.47 to 0.49), and higher if uACR increased by 2-fold (HR from 1.18 to 2.56), irrespective of baseline uACR.
Conclusions: There is substantial risk of adverse kidney outcomes among patients with IgAN and uACR between 0.3 and 1.0 g/g, a population currently considered at low risk of CKD progression. Reduction in uACR is associated with better kidney outcomes, irrespective of baseline uACR.
Keywords: IgA nephropathy; albuminuria; chronic kidney disease; kidney replacement therapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
The authors do not report any additional disclosure in relation to this study. Unrelated to the study, M.E. reports personal honoraria for lectures by AstraZeneca, Astellas Pharma, Vifor Pharma, Fresenius Healthcare, Baxter Healthcare, and being a member of advisory boards for Astellas, AstraZeneca, and Vifor Pharma. J.J.C. reports funding to Karolinska Institutet by AstraZeneca, Astellas, Amgen, Vifor Pharma, and NovoNordisk; personal honoraria for lectures by Fresenius Kabi, Baxter Healthcare, and Abbott, and being a member of advisory boards for Astellas, AstraZeneca, and GSK. S.L. reports personal honoraria for lectures by AstraZeneca and Boehringer Ingelheim and being a member of advisory boards for Boehringer Ingelheim, STADA, and Chinook Therapeutics.
Figures


References
-
- McGrogan A, Franssen CFM, de Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

