Cold-shock proteome of myoblasts reveals role of RBM3 in promotion of mitochondrial metabolism and myoblast differentiation
- PMID: 38688991
- PMCID: PMC11061143
- DOI: 10.1038/s42003-024-06196-4
Cold-shock proteome of myoblasts reveals role of RBM3 in promotion of mitochondrial metabolism and myoblast differentiation
Abstract
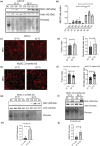
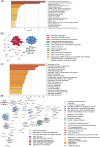
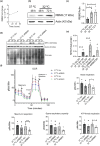
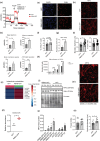
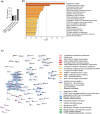
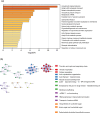
Adaptation to hypothermia is important for skeletal muscle cells under physiological stress and is used for therapeutic hypothermia (mild hypothermia at 32 °C). We show that hypothermic preconditioning at 32 °C for 72 hours improves the differentiation of skeletal muscle myoblasts using both C2C12 and primary myoblasts isolated from 3 month and 18-month-old mice. We analyzed the cold-shock proteome of myoblasts exposed to hypothermia (32 °C for 6 and 48 h) and identified significant changes in pathways related to RNA processing and central carbon, fatty acid, and redox metabolism. The analysis revealed that levels of the cold-shock protein RBM3, an RNA-binding protein, increases with both acute and chronic exposure to hypothermic stress, and is necessary for the enhanced differentiation and maintenance of mitochondrial metabolism. We also show that overexpression of RBM3 at 37 °C is sufficient to promote mitochondrial metabolism, cellular proliferation, and differentiation of C2C12 and primary myoblasts. Proteomic analysis of C2C12 myoblasts overexpressing RBM3 show significant enrichment of pathways involved in fatty acid metabolism, RNA metabolism and the electron transport chain. Overall, we show that the cold-shock protein RBM3 is a critical factor that can be used for controlling the metabolic network of myoblasts.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures

References
-
- Kochanek, P. M. The brain, the heart, and therapeutic hypothermia. Cleve. Clin. J. Med.76, S8–12 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

